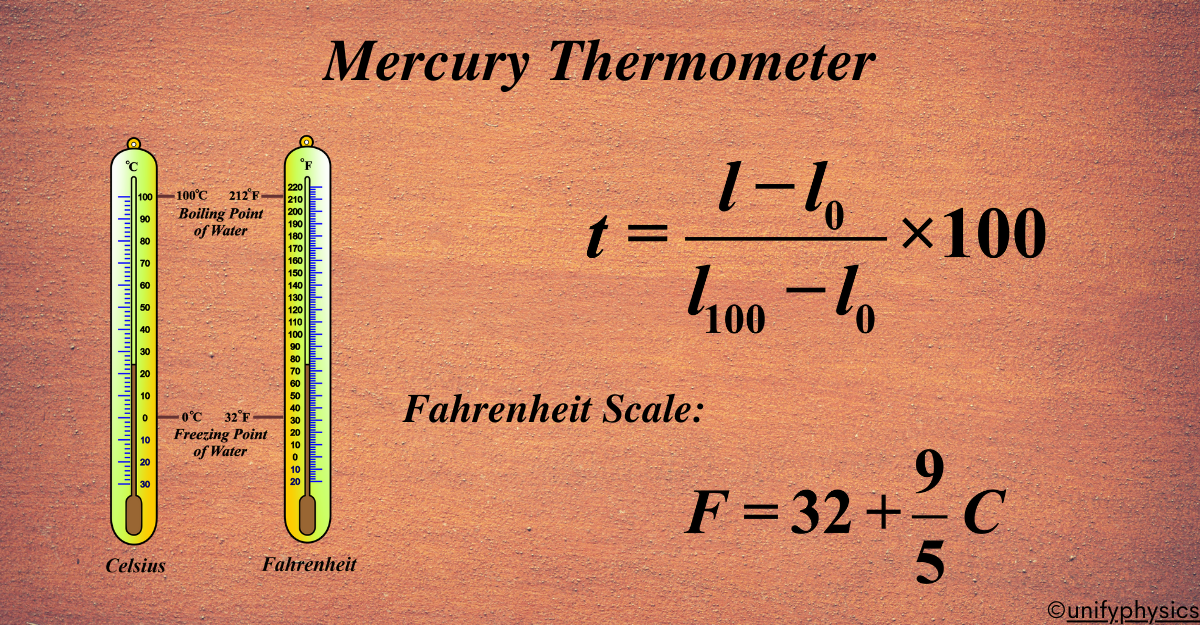
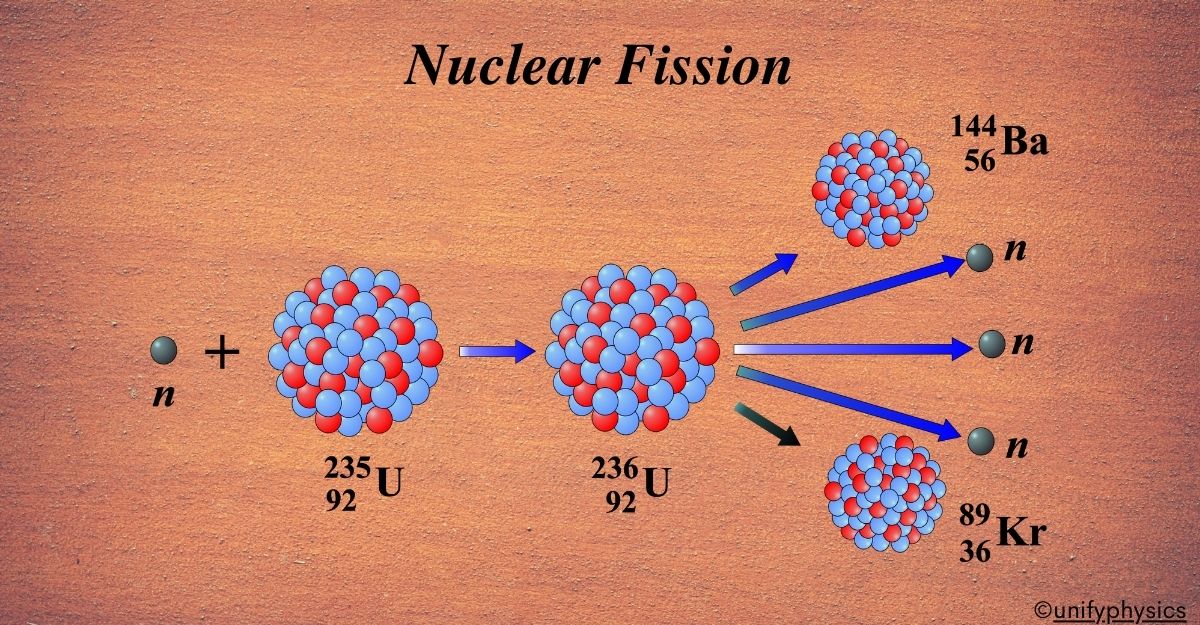
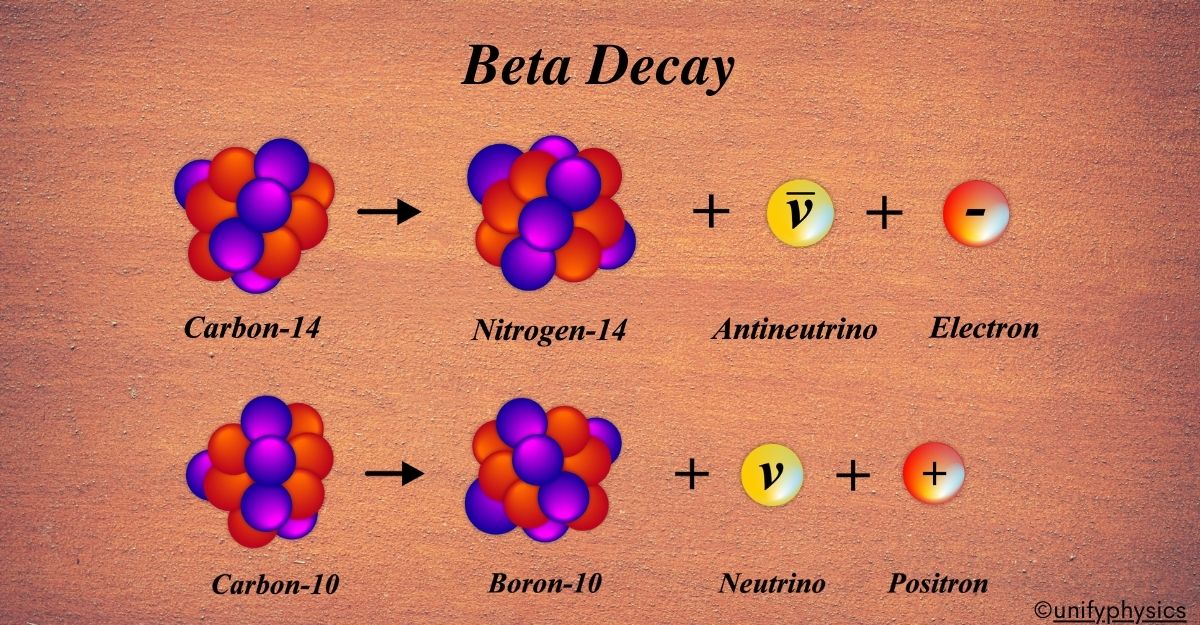
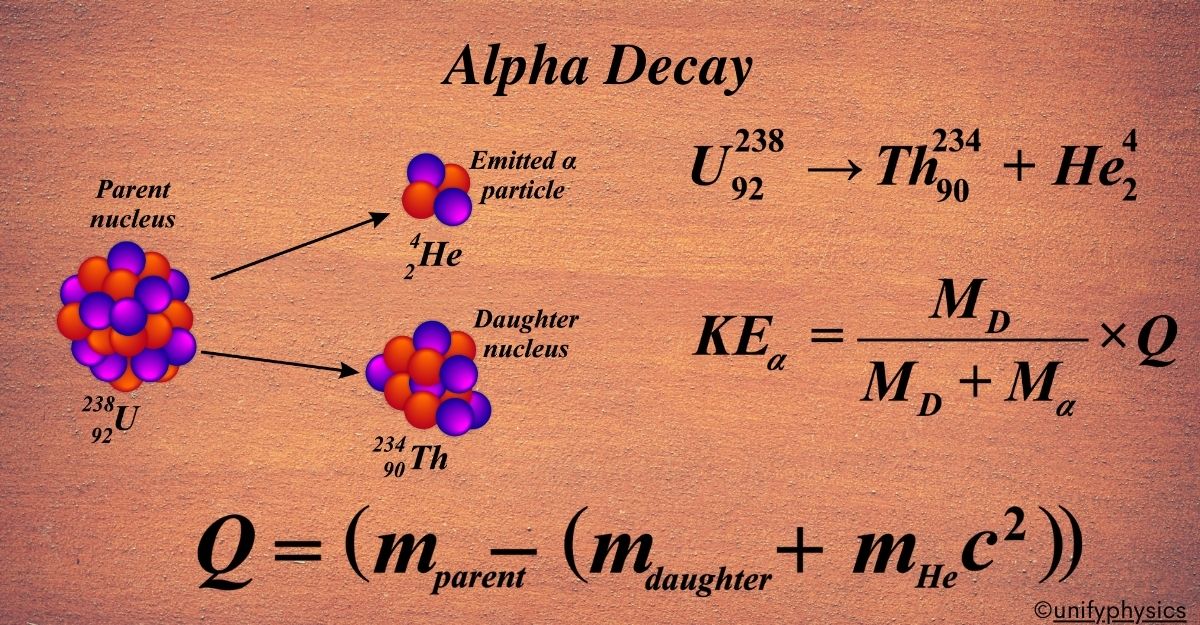
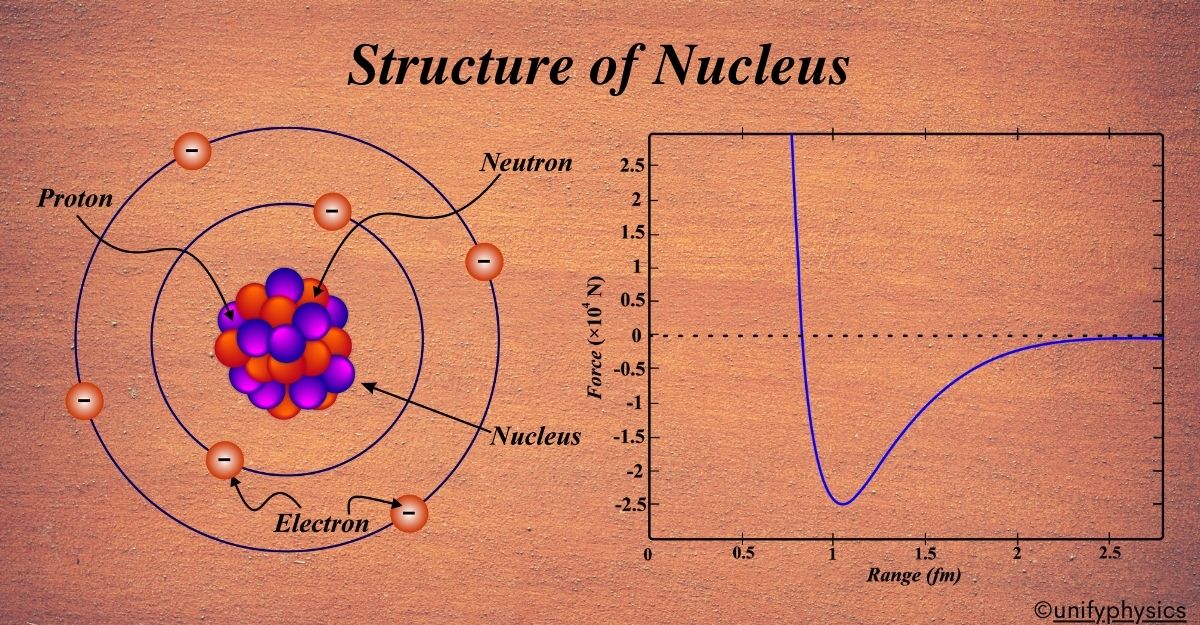
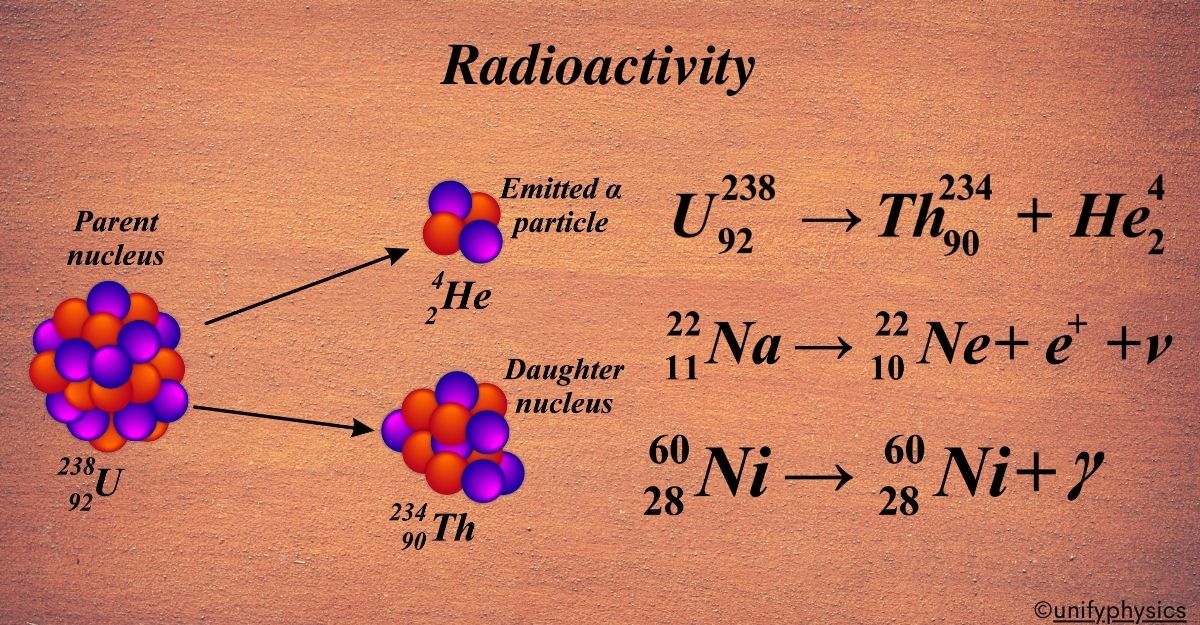
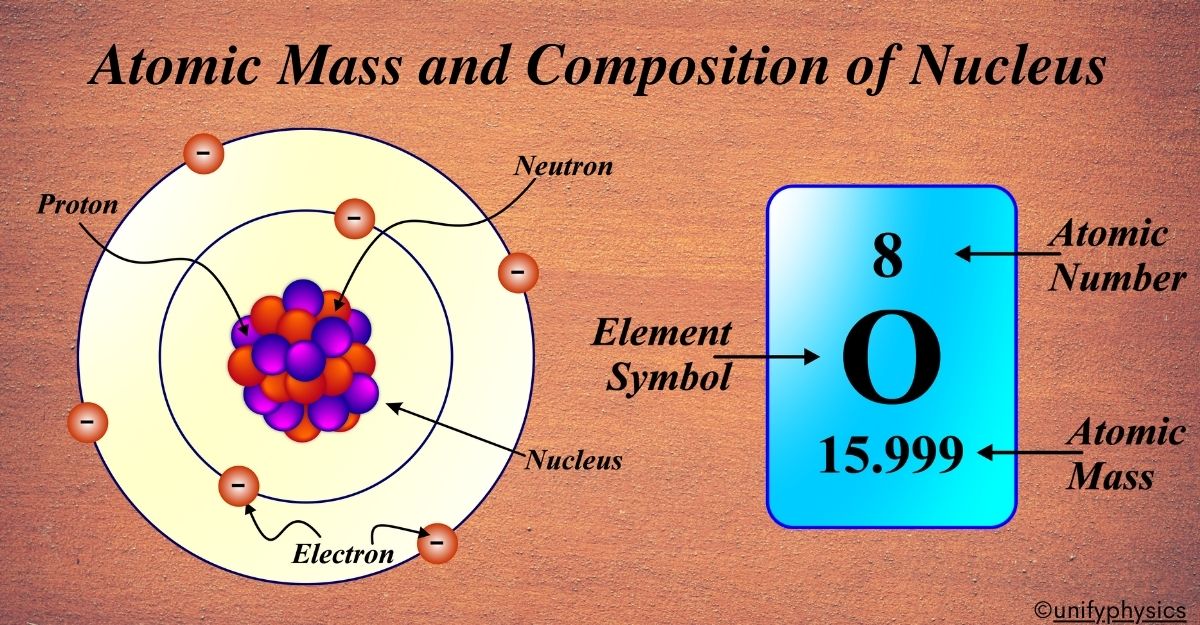
Mercury Thermometers
Temperature is something we all feel intuitively—hot or cold—but measuring it precisely requires a scientific approach. We’ll break this down step by step in a simple, logical order, starting with the history and building up to how we assign numerical values to temperature. I’ll use clear headings, bullet points, and equations to make it easy … Read more