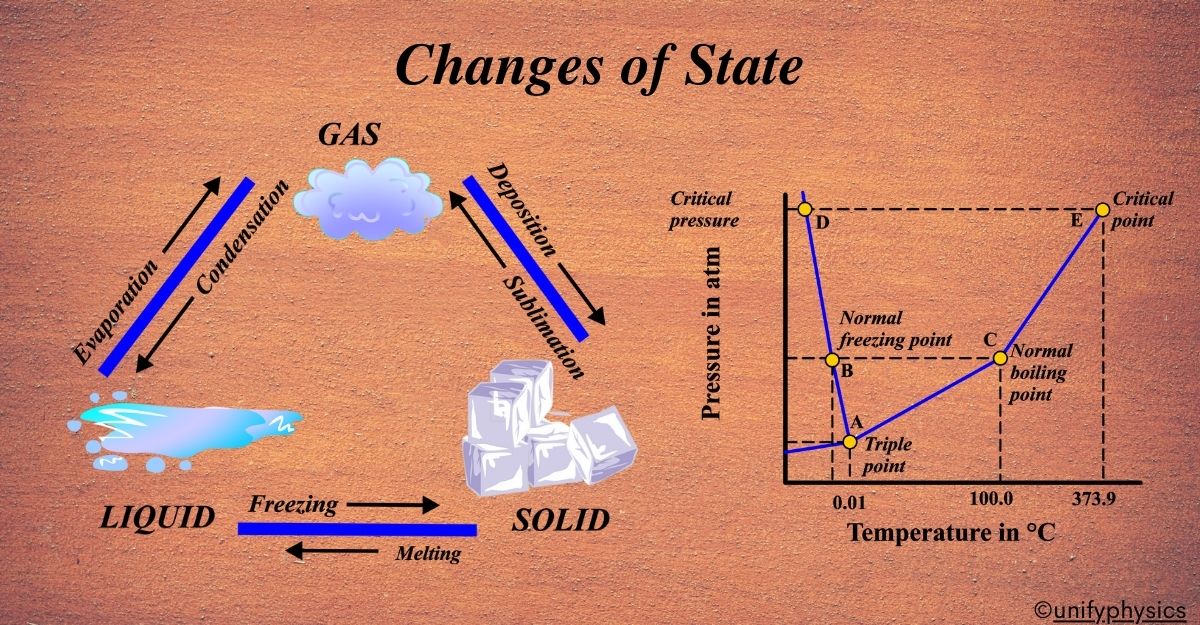
The story of changes of state is a tale of transformation, where substances dance between being solid, liquid, and gas. This story is as old as time itself, but our understanding of it has evolved over centuries.
Ancient Greek philosophers first pondered the idea that matter could change states. They believed in four elements: earth, water, air, and fire, and saw changes of state as transformations between these elements.
Fast forward to the 17th and 18th centuries, during the Scientific Revolution, when scientists like Robert Boyle and Joseph Black began to study changes of state systematically. They started to move away from the idea of elements and towards the concept of matter made up of particles.
In the 18th century, Joseph Black introduced the idea of latent heat, which is the heat absorbed or released during a change of state without a change in temperature¹. This groundbreaking concept helped explain why ice could absorb heat yet not change in temperature as it melted.
Today, we understand changes of state in terms of kinetic theory and intermolecular forces. We know that adding or removing energy can make the particles in a substance move more or less, leading to changes between solid, liquid, and gas.
What are Changes of State?
Imagine observing a block of ice melting into a puddle of water on a warm sunny day or witnessing water vapor condensing into droplets on a cold window pane. These everyday phenomena are examples of changes of state, where substances transition between different physical states, such as solid, liquid, and gas.
Changes of state are fundamental processes governed by the principles of thermodynamics, offering valuable insights into the behavior of matter under varying temperature and pressure conditions. In this exploration, we will embark on a journey to understand the mechanisms behind these transformations, unraveling the mysteries of melting, freezing, vaporization, condensation, sublimation, and deposition.
From the formation of clouds in the sky to the formation of icebergs in the ocean, changes of state shape our environment and drive countless natural processes, making them a captivating subject of study in the realm of physics. Through a combination of real-world examples, simple explanations, and practical applications, we will demystify the concepts of changes of state.
Changes of state, also known as phase transitions, are the transformations that occur when a substance moves from one physical state to another—solid, liquid, or gas. These changes are purely physical, meaning they do not alter the substance’s chemical composition. Instead, they involve a change in the arrangement and energy of the particles that make up the material.
When a substance changes state, its particles either absorb or release energy. This energy is typically in the form of heat. As the energy level of the particles changes, so does their movement and interaction with each other, leading to a change in the physical state. For example, consider water:
- As a solid (ice), water molecules are tightly packed in a structured lattice, vibrating in place but not moving past each other.
- As a liquid, the molecules have more energy and can move around each other freely, though they’re still close together.
- As a gas (steam), the molecules have enough energy to break away from each other and move independently, filling the available space.
These changes can be reversed. For instance, water vapor can condense into liquid water, and liquid water can freeze into ice. The process is cyclical and allows matter to transition between different states depending on the conditions, such as temperature and pressure.
What Causes Phase Changes?
Phase changes, or phase transitions, occur when a substance changes from one state of matter to another, such as from solid to liquid, liquid to gas, or vice versa. When a substance absorbs or loses energy, usually in the form of heat, its particles move differently, leading to a change in state. These changes are driven by two main factors: temperature and pressure.
- Temperature: When a substance is heated, its particles gain kinetic energy, which causes them to vibrate more vigorously and move further apart. If enough energy is absorbed, the particles can overcome the forces holding them together, leading to a phase change. For example, heating ice (solid) will cause it to melt into water (liquid), and further heating water will cause it to vaporize into steam (gas).
- Pressure: Changes in pressure can also induce phase changes. Increasing pressure can force particles closer together, which may change a gas into a liquid or a liquid into a solid. Conversely, reducing pressure can allow particles to move further apart, potentially changing a solid into a liquid or a liquid into a gas.
At the phase transition point, such as the boiling point between liquid and gas phases, the two states of matter have identical free energies and are equally likely to exist. This is why water boils at 100°C (212°F) at sea level (1 atm pressure), but at higher altitudes, where the pressure is lower, it boils at a lower temperature.
Also Read: Heat Capacity
Change of phase between Solids and Liquids
The transition between solid and liquid states involves two key processes: melting and freezing. These processes are the direct result of changes in energy within a substance’s particles.
Melting (Solid to Liquid)
Melting, also known as fusion, occurs when a solid absorbs enough thermal energy to overcome the forces holding its molecules in a fixed position. As the solid heats up, the particles vibrate more vigorously until they have enough energy to move around each other, resulting in a liquid state. The temperature at which this happens is called the melting point. Here’s how it happens:
- Absorption of Energy: As a solid begins to heat up, it absorbs thermal energy, causing the particles within it to vibrate more intensely.
- Disruption of Structure: At a certain point, called the melting point, the particles gain sufficient energy to overcome the attractive forces holding them in place.
- Transition to Liquid: Once these forces are overcome, the particles can move past each other freely, resulting in a liquid state.
The melting point is a specific temperature unique to each substance and is a characteristic physical property. For example, the melting point of ice (water) is 0°C or 32°F at standard atmospheric pressure.
During melting, the substance remains at the melting point temperature until the entire solid has transitioned to a liquid, even though it continues to absorb heat. This absorbed heat, known as the heat of fusion, is used to change the state rather than increase the temperature. It’s important to note that melting is an endothermic process, meaning it absorbs heat from the surroundings. This is why ice melts in your hand; it absorbs heat from your warmer skin.
Freezing (Liquid to Solid)
Freezing is the reverse process of melting. When a liquid loses thermal energy, its particles slow down and begin to arrange themselves into a more structured, fixed pattern, forming a solid. This happens at the freezing point, which, interestingly, is the same temperature as the melting point for a given substance under the same pressure. Here’s a step-by-step explanation:
- Loss of Energy: As a liquid cools down, it loses thermal energy to its surroundings. This loss of energy results in a decrease in the kinetic energy of the particles.
- Formation of Bonds: As the particles lose energy, they move less vigorously and begin to form bonds with each other, creating a structured pattern.
- Solidification: Once the particles are locked into place, the liquid becomes a solid. This occurs at a specific temperature known as the freezing point, which is the same as the melting point for a given substance under the same pressure.
During the freezing process, the temperature of the liquid remains constant at the freezing point until the entire liquid has solidified. This is because the energy being lost is used to change the state of the substance rather than to lower the temperature further.
It’s important to note that freezing is an exothermic process, meaning it releases heat to the surroundings. This is why the surroundings can feel colder when a liquid nearby is freezing. Freezing is not only a common occurrence in nature, such as the formation of ice on a lake in winter but it’s also utilized in various technologies, including food preservation and cryogenics.
These phase changes are not only common in everyday life, such as when water freezes into ice or ice cream melts on a hot day, but they’re also essential in various industrial processes, like the manufacturing of metals and plastics. It’s important to note that the melting and freezing points of a substance can change with pressure. However, at standard atmospheric pressure, these values remain constant for a pure substance and are characteristic properties of that material.
Change of phase between Gases and Liquids
The transition between the gaseous and liquid states of matter is a fascinating process that involves the movement and energy of particles.
Vaporization
Vaporization is the process where a liquid changes into a gas. This phase transition can occur in two different ways: evaporation and boiling. Both are types of vaporization, but they occur under different conditions.
Evaporation: Evaporation is a surface phenomenon that happens at temperatures below the boiling point of the liquid. It occurs when molecules at the surface of the liquid gain enough energy to overcome the intermolecular forces and escape into the air as vapor. This process can happen at any temperature and does not require the liquid to be heated to its boiling point. For example, clothes drying on a line involves evaporation.
Boiling: Boiling is a bulk phenomenon that happens throughout the entire liquid when it reaches its boiling point. At this temperature, bubbles of vapor form within the liquid and rise to the surface. The boiling point is the temperature at which the vapor pressure of the liquid equals the atmospheric pressure. When you boil water in a pot, the formation of bubbles and steam is a visual representation of boiling.
Factors Affecting Vaporization
- Temperature: Higher temperatures increase the kinetic energy of the molecules, making vaporization more likely.
- Surface Area: A larger surface area allows more molecules to escape, increasing the rate of vaporization.
- Pressure: Lower atmospheric pressure makes it easier for molecules to vaporize.
- Wind Speed: Wind can remove vapor from the surface of a liquid, allowing more molecules to escape.
Vaporization is an endothermic process, meaning it requires the absorption of heat. This is why when water evaporates from your skin, you feel cool; the water absorbs heat from your body as it vaporizes.
Condensation
Condensation is the reverse of vaporization. It’s the process where a gas loses enough energy to transform back into a liquid. This usually happens when the gas is cooled or compressed. For example, water vapor in the air condenses to form dew on grass when the temperature drops at night.
When a gas cools, its particles lose kinetic energy and slow down. As the particles lose energy, they move less and their motion becomes more restricted. The decreased movement allows the particles to come closer together, increasing the intermolecular forces between them. Eventually, these forces pull the particles close enough to form a liquid.
Factors Affecting Condensation
- Temperature: The lower the temperature, the more likely condensation will occur, as cooler temperatures reduce the kinetic energy of the gas particles.
- Pressure: Increasing the pressure can also induce condensation by forcing the gas particles closer together.
- Presence of a Surface: Condensation often occurs on surfaces, where the gas comes into contact with a cooler area, facilitating the transition to a liquid.
Examples:
- Dew on Grass: In the morning, you might see dew on grass. This is water vapor in the air that has condensed into liquid water on the cooler surface of the grass.
- Bathroom Mirror Fog: After a hot shower, the bathroom mirror often becomes foggy. This is because the water vapor from the shower condenses on the cooler mirror surface.
Condensation is a critical part of the water cycle, which is essential for life on Earth. It’s also important in various industrial processes, such as distillation, where it’s used to separate mixtures based on differences in volatility.
Both vaporization and condensation are crucial for understanding natural phenomena like the water cycle, as well as for various industrial applications such as distillation and the operation of heat engines. These processes are governed by the kinetic molecular theory, which explains that matter is made up of tiny particles in constant motion. The energy of these particles, along with external conditions like temperature and pressure, determines the state of matter.
Change of phase between gases and solids
This category includes two opposite processes: sublimation and deposition. These phase changes are unique because they involve a direct transition between the solid and gas states, bypassing the liquid state entirely.
Sublimation (Solid to Gas)
Sublimation is the process where a solid changes directly into a gas without passing through the liquid phase. This endothermic phase transition occurs under certain conditions of temperature and pressure, specifically below the triple point of the substance. Here’s how it unfolds:
- Energy Absorption: The solid absorbs energy, which increases the kinetic energy of its particles.
- Overcoming Intermolecular Forces: As the particles gain energy, they begin to vibrate more vigorously. If the solid has sufficient vapor pressure, or if the ambient pressure is low enough, the particles can overcome the intermolecular forces holding them in the solid state.
- Transition to Gas: The particles that gain enough energy escape into the air as gas, completing the transition from solid to gas.
Examples:
- Dry Ice: Perhaps the most well-known example of sublimation is dry ice, which is solid carbon dioxide. At room temperature, dry ice sublimates, turning directly from a solid state into carbon dioxide gas, which is visible as fog.
- Naphthalene: Another common example is naphthalene, found in mothballs. It sublimates at room temperature, releasing its characteristic smell as it transitions into the gas phase.
Applications:
- Forensic Science: Sublimation is used in forensic science, where dye-sublimation printers create detailed and realistic images for analysis.
- Chemistry: Chemists often use sublimation to purify volatile compounds, as it allows the separation of a substance from non-volatile impurities.
Sublimation is an excellent example of how changes in environmental conditions can lead to different states of matter. It also demonstrates the delicate balance of energy and movement that dictates the state of matter.
Deposition (Gas to Solid)
Deposition is the process where a gas changes directly into a solid without first becoming a liquid. This exothermic phase transition occurs under certain conditions, typically involving a decrease in energy or an increase in pressure.
- Energy Release: The gas particles release energy, usually in the form of heat, which decreases their kinetic energy.
- Particle Slowdown: As the particles lose energy, they slow down and their motion becomes more restricted.
- Formation of Solid Structure: Eventually, the particles slow down enough to be captured by the intermolecular forces of nearby particles, leading to the formation of a solid structure.
Examples of Deposition:
- Frost Formation: A common example of deposition is the formation of frost. Water vapor in the air, when exposed to surfaces that are colder than the freezing point of water, will deposit as ice crystals directly onto the surface.
- Snowflakes in Clouds: High in the atmosphere, water vapor can deposit as ice crystals to form snowflakes, bypassing the liquid phase entirely.
Applications:
- Deposition is used in manufacturing processes, such as chemical vapor deposition, to create thin films and coatings on surfaces.
- In cryogenics, gases are often deposited as solids for easier storage and handling.
Deposition is a fascinating process because it demonstrates how a substance can skip an entire phase (liquid) and transition directly to another (solid). It’s a clear example of how changes in environmental conditions, like temperature and pressure, can lead to different states of matter.
Both sublimation and deposition are critical for understanding various natural and industrial processes. For instance, sublimation is responsible for the loss of mass from snowfields and icecaps in the absence of melting. In industry, deposition is used in the manufacture of certain types of thin-film coatings.
These phase changes are governed by the same principles of energy transfer and particle motion that dictate other phase transitions. However, the direct solid-gas transition is unique and provides an excellent example of how changes in environmental conditions can lead to different states of matter.
Phase Diagram
A phase diagram is a graphical way to depict the different states of matter (solid, liquid, and gas) of a substance under various conditions of temperature and pressure. The diagram typically has pressure on the y-axis and temperature on the x-axis. There are three main regions representing the solid, liquid, and gas phases.
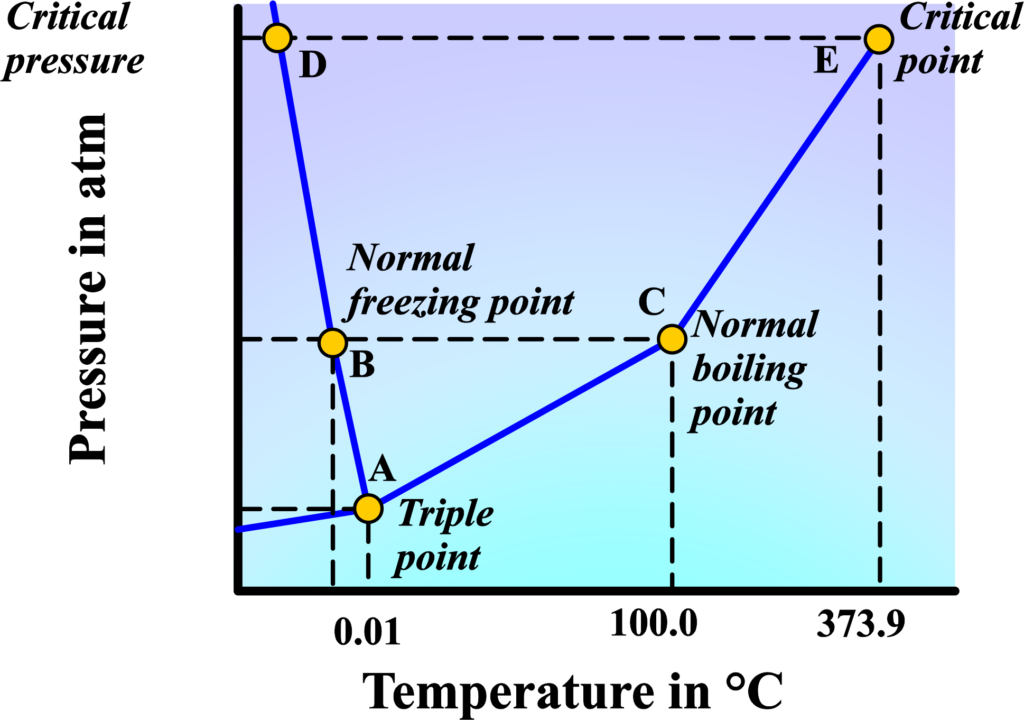
The lines or curves on the diagram represent the boundaries between different phases, known as phase boundaries. The line separating solid and liquid regions is where melting or freezing occurs. The line between liquid and gas regions indicates boiling or condensation points.
The line that separates solid and gas regions shows where sublimation or deposition happens.
Triple Point
The triple point of a substance is a specific combination of temperature and pressure at which three phases—solid, liquid, and gas—coexist in equilibrium. It’s a unique state where all three phases are stable and can transition into each other without changing the overall conditions. This means that the substance can simultaneously display properties of all three states under a specific set of conditions.
Equilibrium
At the triple point, the substance is in a state of dynamic equilibrium. This doesn’t mean that the particles are static; rather, there is a balance in the exchange of particles between the phases. For example, the same number of water molecules that evaporate from the liquid phase will condense back from the gas phase, and the same number that freeze will melt back from the solid phase.
Specific Conditions
The conditions required for a substance to reach its triple point are very precise:
- Temperature: The temperature must be at the exact value where the solid, liquid, and gas phases of the substance are in equilibrium. For water, this temperature is 0.01°C (273.16 K, 32.018 °F).
- Pressure: The pressure must also be exact. For water, the pressure is 611.657 pascals. It’s important to note that this pressure is much lower than atmospheric pressure, which is why we don’t see water in all three phases under normal conditions.
The triple point is significant because it’s a fundamental reference point in thermodynamics. It’s used to define the Kelvin temperature scale and serves as a standard for calibrating temperature measurement devices.
The triple point is used as a reference in thermodynamics and helps define the Kelvin scale of temperature. It’s used to calibrate thermometers and in the study of meteorological phenomena.
Critical Point
Beyond this point, the liquid and gas phases become indistinguishable, and the substance exists as a supercritical fluid. The critical point on a phase diagram represents the end of the line that marks the phase boundary between the liquid and gas states. At this specific point, the properties of the liquid and the gas phases become identical, meaning there is no distinct boundary between them.
Characteristics of the Critical Point:
- Temperature: This is called the critical temperature. Above this temperature, the substance cannot exist as a liquid, regardless of the pressure applied.
- Pressure: This is known as the critical pressure. It is the vapor pressure of the substance at the critical temperature.
- Density: At the critical point, the densities of the liquid and gas phases are equal.
Why is the Critical Point Important?
- Supercritical Fluid: Beyond the critical point, the substance forms what is known as a supercritical fluid, which has properties of both liquids and gases. Supercritical fluids can flow like a gas but dissolve substances like a liquid.
- Industrial Applications: Supercritical fluids are used in various industrial processes, such as supercritical fluid extraction, which is important for decaffeinating coffee and extracting essential oils.
Imagine heating a container of liquid with a gas above it. As you increase the temperature and pressure, the liquid starts to boil, and the gas condenses. However, at the critical point, the distinction between the liquid and gas disappears, and you’re left with a single homogeneous phase – the supercritical fluid.
How to Use a Phase Diagram:
- To determine the state of a substance at a specific temperature and pressure, locate that point on the diagram.
- If the point falls within one of the regions, the substance is in that corresponding state.
- If the point is on a boundary line, the substance is undergoing a phase change.
Example: Water Phase Diagram
- The triple point of water is at approximately 0.01°C and 611.657 pascals, where ice, liquid water, and water vapor coexist.
- The critical point of water is at about 374°C and 22.1 MPa, above which water becomes a supercritical fluid.
Changes of State and Latent Heat
Imagine you have a block of ice, and you want to turn it into water. Now, to do that, you need to add some heat to it. But here’s the interesting part: even after you’ve added enough heat to melt all the ice and turn it into water, the temperature of the water stays the same. Why does that happen?
- Well, it’s because when you’re turning ice into water, you’re not just changing the temperature; you’re also changing the way the water molecules are arranged. In ice, the molecules are all locked in a tight structure, but in water, they’re free to move around.
- So, while you’re melting the ice, the heat you’re adding is being used to break the bonds between the ice molecules. We call this energy “latent heat.” It’s hiding in there, not affecting the temperature, but doing the important job of changing the state of the substance.
- The same thing happens when you’re boiling water. You add heat to turn the water into steam, but again, the temperature stays the same. That’s because the heat is being used to break the bonds between the water molecules, turning them into a gas.
So, latent heat is like the secret energy needed to make state changes happen. It’s the energy that’s hiding in there, doing the work of rearranging molecules, even though you might not see it directly affecting the temperature.
Also Read: Thermal Expansion
Solved Examples
Problem: Calculate the total heat required to convert 100 g of ice at -10°C to steam at 100°C. Specific heat capacity of ice is 2.1 J/g°C, specific heat capacity of water is 4.18 J/g°C, latent heat of fusion of ice is 334 J/g, and latent heat of vaporization of water is 2260 J/g.
Solution:
Heat to raise the temperature of ice from -10°C to 0°C:
\(\displaystyle Q_1 = m \cdot c_{ice} \cdot \Delta T \)
\(\displaystyle Q_1 = 100 \cdot 2.1 \cdot (0 – (-10)) \)
\(\displaystyle Q_1 = 100 \cdot 2.1 \cdot 10 \)
Q1 = 2100 J
Heat to melt the ice at 0°C to water at 0°C:
\(\displaystyle Q_2 = m \cdot L_f \)
\(\displaystyle Q_2 = 100 \cdot 334 \)
Q2 = 33400 J
Heat to raise the temperature of water from 0°C to 100°C:
\(\displaystyle Q_3 = m \cdot c_{water} \cdot \Delta T \)
\(\displaystyle Q_3 = 100 \cdot 4.18 \cdot (100 – 0) \)
\(\displaystyle Q_3 = 100 \cdot 4.18 \cdot 100 \)
Q3 = 41800 J
Heat to convert water at 100°C to steam at 100°C:
\(\displaystyle Q_4 = m \cdot L_v \)
\(\displaystyle Q_4 = 100 \cdot 2260 \)
Q4 = 226000 J
Total heat required:
\(\displaystyle Q_{total} = Q_1 + Q_2 + Q_3 + Q_4 \)
\(\displaystyle Q_{total} = 2100 + 33400 + 41800 + 226000 \)
Qtotal = 303300 J
The total heat required is 303300 J.
Problem 2: Calculate the heat required to sublimate 50 g of dry ice (solid CO₂) at -78.5°C to CO₂ gas at 0°C. The latent heat of sublimation for CO₂ is 571 J/g, and the specific heat capacity of CO₂ gas is 0.839 J/g°C.
Solution:
Heat to sublimate solid CO₂ to CO₂ gas at -78.5°C:
\(\displaystyle Q_1 = m \cdot L_s \)
\(\displaystyle Q_1 = 50 \cdot 571 \)
\(\displaystyle Q_1 = 28550 \, J \)
Heat to raise the temperature of CO₂ gas from -78.5°C to 0°C:
\(\displaystyle Q_2 = m \cdot c_{CO2} \cdot \Delta T \)
\(\displaystyle Q_2 = 50 \cdot 0.839 \cdot (0 – (-78.5)) \)
\(\displaystyle Q_2 = 50 \cdot 0.839 \cdot 78.5 \)
\(\displaystyle Q_2 = 3290.575 \, J \)
Total heat required:
\(\displaystyle Q_{total} = Q_1 + Q_2 \)
\(\displaystyle Q_{total} = 28550 + 3290.575 \)
\(\displaystyle Q_{total} = 31840.575 \, J \)
The total heat required is 31840.575 J.
Problem 3: Calculate the amount of heat required to change 10 g of ice at the triple point of water (0.01°C) to steam at the same temperature. Given that the latent heat of fusion for water is 334 J/g and the latent heat of vaporization is 2260 J/g.
Solution:
Heat to melt the ice into water:
\(\displaystyle Q_1 = m \cdot L_f \)
\(\displaystyle Q_1 = 10 \cdot 334 \)
\(\displaystyle Q_1 = 3340 \, J \)
Heat to convert water to steam:
\(\displaystyle Q_2 = m \cdot L_v \)
\(\displaystyle Q_2 = 10 \cdot 2260 \)
\(\displaystyle Q_2 = 22600 \, J\)
Total heat required:
\(\displaystyle Q_{total} = Q_1 + Q_2 \)
\(\displaystyle Q_{total} = 3340 + 22600 \)
\(\displaystyle Q_{total} = 25940 \, J \)
The total heat required is 25940 J.
Problem 4: A substance has a critical temperature of 374°C and a critical pressure of 218 atm. Calculate the energy required to convert 1 kg of the substance from liquid at 374°C and 218 atm to gas at the same temperature and pressure. The latent heat of vaporization at the critical point is 2000 J/g.
Solution:
- Mass (m) = 1 kg = 1000 g
- Latent heat of vaporization (Lv) = 2000 J/g
Heat required:
\(\displaystyle Q = m \cdot L_v \)
\(\displaystyle Q = 1000 \cdot 2000 \)
Q = 2000000 J
The energy required is 2000000 J.
Problem 5: Calculate the total heat required to convert 200 g of ethanol from solid at -114.5°C to vapor at 78.4°C. Specific heat capacities are 2.44 J/g°C for solid ethanol, 2.3 J/g°C for liquid ethanol, and the latent heats are 109 J/g for fusion and 841 J/g for vaporization.
Solution:
Heat to raise the temperature of solid ethanol from -114.5°C to -114.5°C:
\(\displaystyle Q_1 = m \cdot c_{solid} \cdot \Delta T \)
\(\displaystyle Q_1 = 200 \cdot 2.44 \cdot (0 – (-114.5)) \)
\(\displaystyle Q_1 = 200 \cdot 2.44 \cdot 114.5 \)
\(\displaystyle Q_1 = 55868 \, J \)
Heat to melt the solid ethanol:
\(\displaystyle Q_2 = m \cdot L_f \)
\(\displaystyle Q_2 = 200 \cdot 109 \)
\(\displaystyle Q_2 = 21800 \, J \)
Heat to raise the temperature of liquid ethanol from 0°C to 78.4°C:
\(\displaystyle Q_3 = m \cdot c_{liquid} \cdot \Delta T \)
\(\displaystyle Q_3 = 200 \cdot 2.3 \cdot (78.4 – 0) \)
\(\displaystyle Q_3 = 200 \cdot 2.3 \cdot 78.4 \)
\(\displaystyle Q_3 = 36088 \, J \)
Heat to vaporize the liquid ethanol:
\(\displaystyle Q_4 = m \cdot L_v \)
\(\displaystyle Q_4 = 200 \cdot 841 \)
\(\displaystyle Q_4 = 168200 \, J \)
Total heat required:
\(\displaystyle Q_{total} = Q_1 + Q_2 + Q_3 + Q_4 \)
\(\displaystyle Q_{total} = 55868 + 21800 + 36088 + 168200 \)
\(\displaystyle Q_{total} = 281956 \, J \)
The total heat required is 281956 J.
Problem 6: Calculate the total heat removed when 500 g of water at 30°C is cooled to -10°C. Given specific heat capacities: water = 4.18 J/g°C, ice = 2.1 J/g°C; latent heat of fusion = 334 J/g.
Solution:
Heat to cool water from 30°C to 0°C:
\(\displaystyle Q_1 = m \cdot c_{water} \cdot \Delta T \)
\(\displaystyle Q_1 = 500 \cdot 4.18 \cdot (0 – 30) \)
\(\displaystyle Q_1 = 500 \cdot 4.18 \cdot (-30) \)
\(\displaystyle Q_1 = -62700 \, J \)
Heat to freeze water at 0°C to ice at 0°C:
\(\displaystyle Q_2 = m \cdot L_f \)
\(\displaystyle Q_2 = 500 \cdot 334 \)
\(\displaystyle Q_2 = -167000 \, J \)
Heat to cool ice from 0°C to -10°C:
\(\displaystyle Q_3 = m \cdot c_{ice} \cdot \Delta T \)
\(\displaystyle Q_3 = 500 \cdot 2.1 \cdot (-10 – 0) \)
\(\displaystyle Q_3 = 500 \cdot 2.1 \cdot (-10) \)
\(\displaystyle Q_3 = -10500 \, J \)
Total heat removed:
\(\displaystyle Q_{total} = Q_1 + Q_2 + Q_3 \)
\(\displaystyle Q_{total} = -62700 – 167000 – 10500 \)
\(\displaystyle Q_{total} = -240200 \, J \)
The total heat removed is 240200 J.
FAQs
What are the different states of matter?
The different states of matter are solid, liquid, gas, and plasma. Solids have a fixed shape and volume, liquids have a fixed volume but take the shape of their container, gases have neither fixed volume nor shape, and plasma is an ionized state of matter found in high-energy environments like stars.
What is the process of changing from solid to liquid called?
The process of changing from solid to liquid is called melting. During melting, a substance absorbs heat, causing its molecules to vibrate more vigorously until they overcome their rigid structure and transition into a liquid state.
How does a liquid change into a gas?
A liquid changes into a gas through a process called vaporization, which includes both evaporation and boiling. Evaporation occurs at the surface of the liquid at temperatures below the boiling point, while boiling occurs throughout the liquid when it reaches its boiling point.
What is sublimation?
Sublimation is the process in which a solid changes directly into a gas without passing through the liquid state. Common examples include dry ice (solid carbon dioxide) sublimating into carbon dioxide gas and snow disappearing in cold weather without melting.
How does a gas change into a liquid?
A gas changes into a liquid through condensation. During condensation, a gas loses energy in the form of heat, causing its molecules to slow down and come closer together, forming a liquid. This process is commonly observed as water vapor condensing into dew on a cold surface.
What factors affect the rate of evaporation?
The rate of evaporation is affected by several factors, including temperature (higher temperatures increase evaporation rates), surface area (larger surface areas allow more molecules to escape), humidity (lower humidity increases evaporation), and wind speed (faster winds remove vapor molecules more quickly).
What is the significance of latent heat in changes of state?
Latent heat is the heat required to change the state of a substance without changing its temperature. It is significant because it explains why temperature remains constant during phase transitions, such as melting or boiling, despite the continuous input or release of heat. This concept is crucial in understanding energy transfer during changes of state.