The story of water’s peculiar behavior begins in the mid-19th century. In 1859, a British scientist named W.J.M. Rankine made a groundbreaking discovery. He observed that when water freezes, it expands and occupies more space than it does in its liquid form. This was contrary to the common behavior of substances, which typically contract upon freezing.
Rankine’s discovery was pivotal because it challenged the existing understanding of how matter behaves in different states. Until then, it was widely accepted that substances contract when they solidify because their particles are expected to come closer together as they lose energy.
However, water proved to be an exception to this rule. As the water cools and approaches the freezing point, instead of continuing to contract, it begins to expand below 4°C. This expansion upon freezing is what we refer to as the “anomalous expansion of water.”
This anomaly was further studied and explained through the unique molecular structure of water. The hydrogen bonds between water molecules create a lattice-like structure when water turns into ice, causing it to take up more space.
Understanding the historical context of the anomalous expansion of water helps students appreciate the evolution of scientific thought and the importance of challenging assumptions with empirical evidence. It’s a testament to the curiosity and persistence of scientists like Rankine, who help us unravel the mysteries of the natural world.
Anomalous Expansion of Water
Most substances expand when heated and contract when cooled. However, water is exceptional. When water is cooled down to 4°C, it contracts like other liquids, reaching its maximum density at this temperature. But, as it cools further from 4°C down to 0°C, it begins to expand. This is known as the anomalous expansion of water.
In most substances, heating leads to expansion, and cooling results in contraction. Water, however, behaves a bit differently due to its unique molecular structure. Here’s what happens:
Normal Behavior: As you heat most liquids, their molecules move faster and spread apart, causing the liquid to expand. When cooled, the molecules slow down and come closer together, resulting in contraction.
Water’s Anomaly: Water follows the normal rule only until it is cooled down to about 4°C. At this temperature, water reaches its maximum density and is at its smallest volume. But as water cools further from 4°C down to 0°C, it begins to expand.
Molecular Explanation: This unusual behavior is due to the hydrogen bonds in water molecules. At temperatures above 4°C, water molecules are close together. But as water cools to 4°C and below, the hydrogen bonds cause the molecules to arrange in a specific structure that takes up more space.
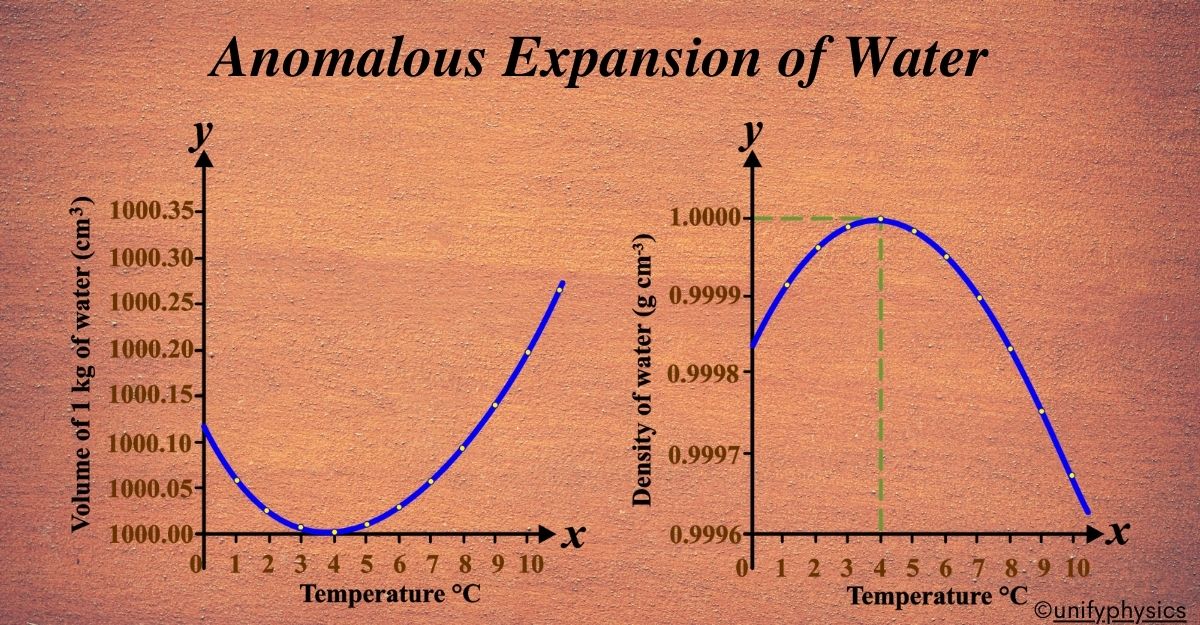
Graphical Representation: If we plot the density of water against temperature, we’ll see that the density increases as the temperature drops to 4°C, then decreases from 4°C to 0°C. This is the opposite of what we’d expect and is why it’s called “anomalous.”
Imagine a graph where the x-axis represents temperature and the y-axis represents the density of water. As we move from left to right on the graph, the temperature increases, and as we move up and down, the density increases or decreases respectively.
- Starting Point (0°C): At 0°C, water is in its solid form as ice. Here, the graph starts with the density of ice, which is less than the density of liquid water.
- As Temperature Increases (0°C to 4°C): As the temperature rises from 0°C towards 4°C, the density of water increases. This is where water behaves anomalously compared to most substances. Instead of expanding, it contracts, becoming denser until it reaches 4°C.
- Maximum Density Point (4°C): At exactly 4°C, water reaches its maximum density. This is the peak of the graph. It’s the point where water is the heaviest and occupies the least volume.
- Beyond 4°C (4°C to 100°C): As the temperature continues to increase beyond 4°C, the density of water starts to decrease normally, just like other liquids. The graph slopes downward, indicating that water expands and becomes less dense as it gets warmer.
When the temperature of water continues to increase beyond 4°C, the behavior of water’s volume changes from its anomalous state back to normal.
- Above 4°C: As the temperature rises above 4°C, water starts behaving like most other substances. The molecules gain more kinetic energy and begin to move apart.
- Expansion: This increase in molecular movement causes the water to expand. The volume of water increases as the temperature rises.
- Density Decreases: With the expansion of volume, the density of water decreases. This means that the same amount of water will occupy more space at higher temperatures.
- Graphically: On a graph plotting temperature against volume, you would see a curve that initially dips down as the temperature goes from 0°C to 4°C, indicating the anomalous contraction. Then, as the temperature rises above 4°C, the curve would rise, showing the expansion of water.
This graphical representation is crucial because it visually explains the unique behavior of water. It shows that water has a maximum density at 4°C and that it expands upon cooling from 4°C down to 0°C, which is the opposite of what happens with most other substances. This anomaly is why ice floats on water and has significant implications for life on Earth, especially in aquatic environments.
Critical Point: The critical temperature here is 4°C. It’s the turning point where the water stops behaving like a typical liquid and starts exhibiting its anomalous expansion.
Why is Water Unique?
Water’s uniqueness lies in its molecular structure. A water molecule consists of one oxygen atom bonded to two hydrogen atoms. The shape of the molecule and the hydrogen bonds between water molecules cause this unusual behavior. When water cools below 4°C, the hydrogen bonds adjust to hold the molecules further apart, making ice less dense than liquid water.
Molecular Structure: Water’s uniqueness starts at the molecular level. Each water molecule is made up of two hydrogen atoms bonded to one oxygen atom. The shape of the molecule and the way the atoms are bonded give water some remarkable properties.
Hydrogen Bonding: The hydrogen atoms in a water molecule form weak bonds with oxygen atoms in other water molecules. These are called hydrogen bonds, and they play a crucial role in water’s behavior. At normal temperatures, these bonds keep water in a liquid state, with the molecules close together but still moving around.
Behavior Below 4°C: As the water cools below 4°C, these hydrogen bonds cause the molecules to arrange themselves in a more structured form. This structure takes up more space than when the molecules are moving freely, which is why water expands as it cools from 4°C down to 0°C.
Density Anomaly: Typically, as substances cool, they become denser because their molecules get closer together. Water, however, becomes densest at 4°C and then begins to expand, becoming less dense as it approaches the freezing point. This is the opposite of what happens with most substances and is what we refer to as the anomalous expansion of water.
Implications for Life: This anomaly has profound implications for life on Earth. If water behaved like other substances, ice would sink, which could drastically alter the climate and make life in cold regions nearly impossible. Instead, ice floats, creating an insulating layer that protects aquatic life during freezing temperatures.
Example: Let’s consider a pond with fish swimming in it during both summer and winter.
During the summer, when the temperature is relatively high, the water in the pond is warmer. As a result, the water molecules are moving more rapidly and are spaced further apart. This causes the water to expand, providing more space for the fish to swim comfortably.
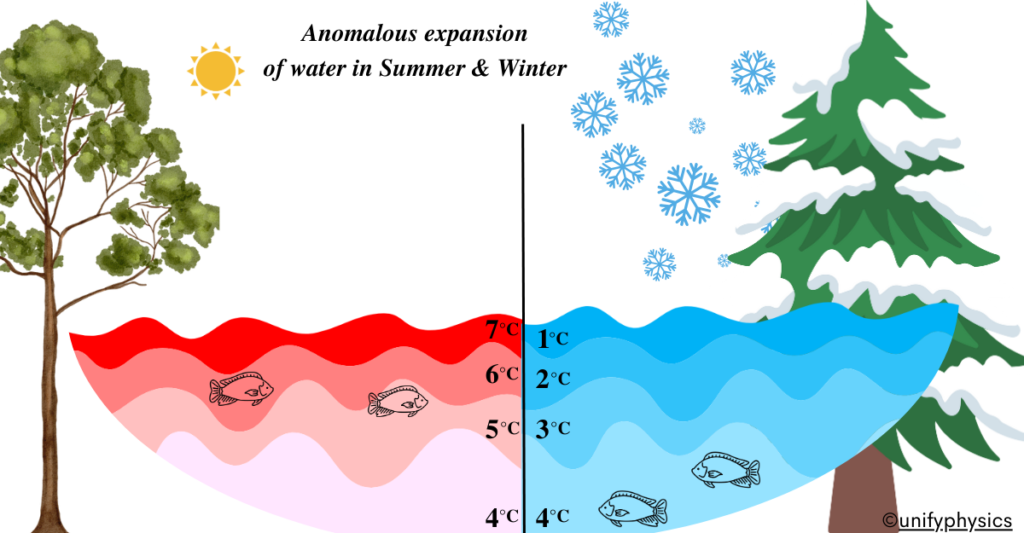
Conversely, during the winter, when the temperature drops, the water in the pond cools down. As the temperature approaches and drops below 4°C (39.2°F), which is the temperature at which water reaches its maximum density, the behavior of water begins to change. At temperatures below 4°C, water molecules start forming a unique arrangement that allows them to come closer together, causing the water to contract and become denser.
This anomalous behavior of water is crucial for the survival of aquatic life during the winter months. As the water cools and contracts, it becomes denser, causing it to sink to the bottom of the pond. This allows the warmer water from below to rise and remain near the surface, creating a more hospitable environment for fish and other aquatic organisms.
If water behaved like most substances and continued to contract as it cooled below 4°C, ice would sink to the bottom of bodies of water, which could have catastrophic effects on aquatic ecosystems. However, because of water’s anomalous expansion, ice floats on the surface, insulating the water below and providing a buffer against extreme cold temperatures.
The anomalous expansion of water plays a crucial role in maintaining the stability and health of aquatic ecosystems, ensuring the survival of fish and other aquatic organisms during both summer and winter seasons.
Properties of Water
Water is not just essential for life but also has some unique physical and chemical properties that are fascinating from a physics perspective:
- Molecular Structure: Water (H2O) is composed of two hydrogen atoms bonded to one oxygen atom. The arrangement of these atoms and the polar nature of the molecule give water its unique properties.
- Hydrogen Bonding: The hydrogen atoms in water molecules form weak bonds with oxygen atoms in other water molecules, known as hydrogen bonds. These bonds are responsible for many of water’s special properties, including its high surface tension and its ability to dissolve many substances.
- High Specific Heat: Water has a high specific heat capacity, which means it can absorb or release a large amount of heat with little temperature change. This property plays a critical role in Earth’s climate and in the body’s ability to regulate temperature.
- High Heat of Vaporization: Water requires a significant amount of energy to change from liquid to gas, which is why sweating is an effective way to cool down. This property also influences weather patterns and climate.
- Density and Expansion: Most substances become denser as they cool, but water reaches its maximum density at 4°C. Below this temperature, water expands as it freezes. This anomalous expansion is due to the way hydrogen bonds arrange water molecules in a hexagonal lattice, which takes up more space than when the molecules are closer together in liquid form.
- Amphoteric Nature: Water can act as both an acid and a base, making it amphoteric. This property is important in many chemical reactions, including those in biological systems.
- Solvent Abilities: Water is often called the “universal solvent” because it can dissolve more substances than any other liquid. This is due to its polarity and ability to form hydrogen bonds, which allows it to interact with various molecules.
These properties of water are not just academic curiosities; they have profound implications for the environment, weather, and life on Earth. For instance, the anomalous expansion of water ensures that ice floats, providing insulation for aquatic life during cold periods. The high heat capacity of water stabilizes ocean temperatures, which affects global climate patterns.
Also Read: Thermal Expansion
Applications
- Preservation of Aquatic Ecosystems: During winter, the top layer of water bodies cools down and reaches 4°C, becoming denser and sinking. This cycle continues until the surface water reaches 0°C. Instead of sinking, this water expands and turns into ice, forming an insulating layer. This ice layer floats on top, preventing the entire water body from freezing and allowing aquatic life to survive in the liquid water below.
- Weathering of Rocks: Water can seep into the cracks and crevices of rocks. When the temperature drops below 4°C, water expands as it freezes, exerting pressure on the rock. This process can cause the rock to crack and break apart over time, a natural process known as frost weathering or freeze-thaw.
- Pipeline Leakage: In regions with cold climates, water pipelines must be designed to accommodate the expansion of water as it freezes. If not, the expanding ice can cause the pipes to burst, leading to leaks and significant damage.
Solved Examples
Example 1: Explain why water exhibits anomalous expansion behavior.
Solution: Water exhibits anomalous expansion behavior due to the presence of hydrogen bonds between water molecules. Unlike most substances, which contract when cooled and expand when heated, water contracts as it cools down from 4°C to 0°C. This is because, at temperatures above 4°C, the thermal motion of water molecules disrupts the hydrogen bonds, causing water to behave like a normal liquid and expand when heated. However, as the water cools below 4°C, the hydrogen bonds start to form a network structure, which results in a decrease in volume despite a decrease in temperature. At 0°C, this network structure reaches its maximum stability, leading to the anomalous expansion behavior of water.
Example 2: Discuss the importance of the anomalous expansion of water in nature.
Solution: The anomalous expansion of water is crucial for various natural phenomena and life forms. For example:
- It prevents bodies of water, such as lakes and ponds, from freezing solid in cold climates, as the ice formed at the surface insulates the water below, allowing aquatic life to survive.
- It regulates the climate by moderating temperature changes in large bodies of water, such as oceans, which affects global weather patterns.
- It supports life by providing a stable environment for aquatic organisms during winter months.
Overall, the anomalous expansion of water plays a vital role in maintaining ecological balance and supporting life on Earth.
Example 3: Calculate the density of water at 4°C and 0°C. Given: Density of water at 4°C = 1000 kg/m³, Coefficient of volume expansion of water = \(\displaystyle 2.1 \times 10^{-4} \, °C^{-1} \).
Solution: Density of water at 4°C: \(\displaystyle \rho_4 = 1000 \, kg/m³ \)
The density of water at 0°C: Using the formula for volume expansion:
\(\displaystyle \frac{\Delta V}{V_0} = \beta \Delta T \)
\(\displaystyle \Delta T = 4°C \)
\(\displaystyle \Delta V = V_0 \beta \Delta T \)
\(\displaystyle V_0 = 1 \, m³ \) (assumed for simplicity)
\(\displaystyle \Delta V = 1 \times (2.1 \times 10^{-4}) \times 4 \)
\(\displaystyle \Delta V = 8.4 \times 10^{-4} \, m³ \)
\(\displaystyle V_0 + \Delta V = 1 + 8.4 \times 10^{-4} \)
\(\displaystyle V = 1.00084 \, m³ \)
\(\displaystyle \rho_0 = \frac{m}{V} = \frac{1000}{1.00084} \)
\(\displaystyle \rho_0 ≈ 999.16 \, kg/m³ \)
Therefore, the density of water at 0°C is approximately ( 999.16 kg/m³).
Example 4: A water pipe with a length of 10m is initially at a temperature of 20°C. If the temperature drops to 10°C, calculate the change in the length of the pipe. Given: Coefficient of linear expansion of water = \(\displaystyle 2.1 \times 10^{-4} \, °C^{-1} \).
Solution: Change in length (∆L) can be calculated using the formula for linear expansion:
\(\displaystyle \Delta L = L_0 \alpha \Delta T \)
\(\displaystyle \Delta T = 20°C – 10°C = 10°C \)
( L0 = 10m )
\(\displaystyle \alpha = 2.1 \times 10^{-4} \, °C^{-1} \)
\(\displaystyle\Delta L = 10 \times (2.1 \times 10^{-4}) \times 10 \)
\(\displaystyle \Delta L = 2.1 \times 10^{-3} \, m \)
Therefore, the change in length of the pipe is \(\displaystyle 2.1 \times 10^{-3} \, m \).
FAQs
What is the anomalous expansion of water, and how does it differ from normal behavior?
The anomalous expansion of water refers to its unusual property of expanding when cooled below 4°C and reaching its maximum density at 4°C. This behavior is contrary to most substances, which contract and become denser as they cool below their freezing point.
Can you explain why water exhibits anomalous expansion?
Water exhibits anomalous expansion due to the unique arrangement of its molecules in the liquid state. As water cools below 4°C, hydrogen bonding causes the molecules to form open hexagonal structures, which take up more space compared to the more densely packed structure at higher temperatures. This results in an increase in volume and a decrease in density.
How does the anomalous expansion of water affect aquatic ecosystems and life forms?
The anomalous expansion of water is essential for the survival of aquatic life forms. Bodies of water freeze from the top down, allowing aquatic organisms to survive in liquid water beneath the surface during winter. If water behaved like most substances and contracted upon freezing, ice would sink, potentially freezing entire bodies of water and disrupting ecosystems.
What are some practical applications of understanding the anomalous expansion of water?
Understanding the anomalous expansion of water has practical applications in various fields. For example, it is crucial in designing plumbing systems to prevent pipes from bursting when water freezes. It also plays a role in meteorology, oceanography, and climate science, influencing phenomena such as ocean currents, weather patterns, and climate dynamics.
Can you describe experiments or demonstrations illustrating the anomalous expansion of water?
One common demonstration involves filling a container with water and inserting a thermometer. As the water cools below 4°C, the temperature decreases, but at 4°C, it temporarily rises before continuing to decrease. This temporary rise in temperature at 4°C indicates the maximum density point and illustrates the anomalous expansion of water.
How does the anomalous expansion of water impact the thermal regulation of aquatic environments?
The anomalous expansion of water plays a crucial role in moderating temperature fluctuations in aquatic environments. Bodies of water act as natural thermal regulators, absorbing heat during the day and releasing it at night. This helps to maintain stable temperatures, supporting diverse aquatic ecosystems and biodiversity.
Are there any industrial or engineering challenges associated with the anomalous expansion of water?
Yes, the anomalous expansion of water can pose challenges in certain engineering and industrial applications. For example, it can lead to the cracking of concrete structures subjected to freezing and thawing cycles, as water expands upon freezing. Engineers must account for this behavior in construction design and materials selection to ensure structural integrity.