The idea of atoms as the fundamental building blocks of matter dates back to ancient Greece, but it wasn’t until the 19th century that the concept began to take a scientific form. John Dalton, in the early 1800s, proposed that each element is composed of unique atoms and that chemical reactions involve the rearrangement of these atoms.
In 1897, J.J. Thomson’s experiments with cathode rays led to the discovery of the electron, a tiny particle with a negative charge. This was a significant breakthrough because it showed that atoms were not indivisible; they contained smaller particles.
Following his discovery, Thomson proposed the “plum pudding” model of the atom. He suggested that the atom was a sphere of positive charge with electrons (the “plums”) scattered throughout, like raisins in a pudding.
The plum pudding model was widely accepted until Ernest Rutherford’s landmark experiment in 1911. Rutherford and his colleagues, Hans Geiger and Ernest Marsden, directed a beam of alpha particles at a thin sheet of gold foil. They observed that while most particles passed straight through, some were deflected at large angles.
Rutherford’s observations contradicted the plum pudding model and led him to propose a new model of the atom. He concluded that the atom must have a small, dense, positively charged center where most of the mass is concentrated. This center was called the nucleus, and it was surrounded by a cloud of electrons.
With the nucleus identified, scientists then discovered the particles it contained. Protons, with a positive charge, were identified by Rutherford in 1919. Neutrons, with no charge, were discovered by James Chadwick in 1932. The discoveries of the electron, proton, and neutron laid the foundation for the modern atomic model. Today, we understand the nucleus to be composed of protons and neutrons, with electrons orbiting in the space around it.
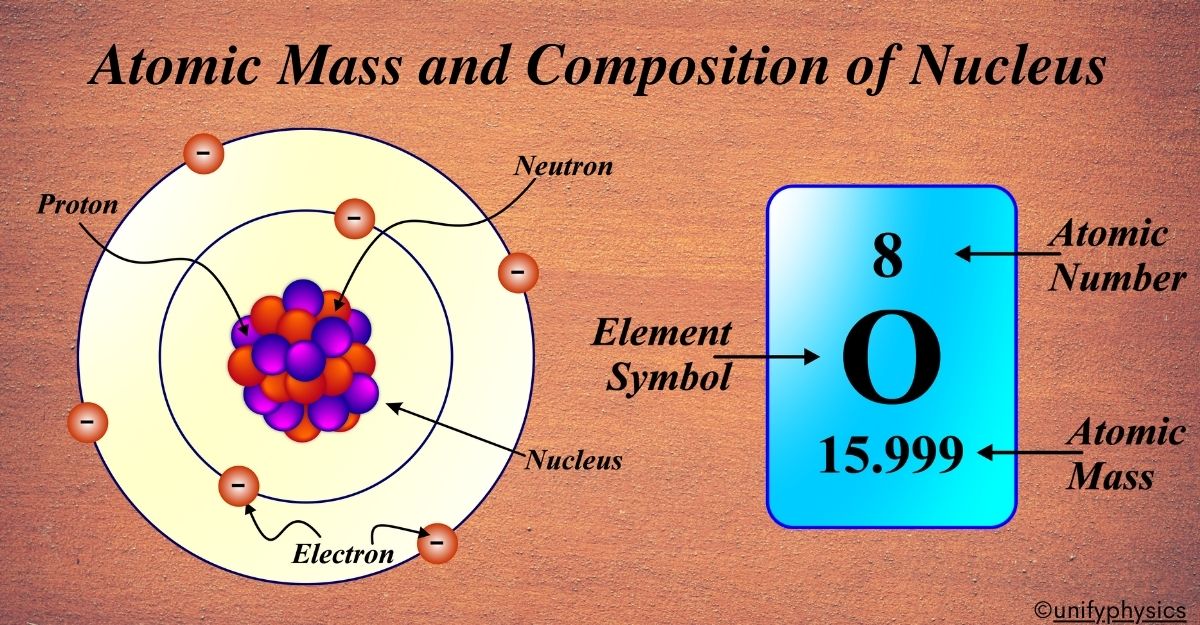
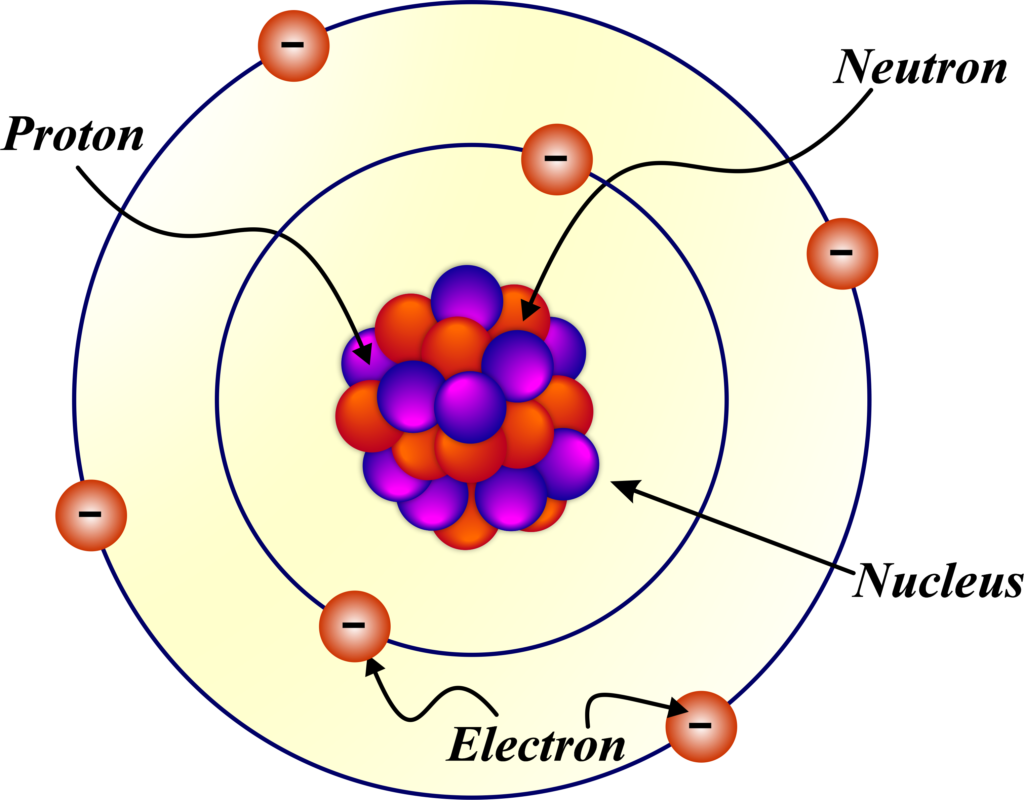
Structure of Atom
Atoms are the basic units of matter and consist of three main particles:
Protons: Positively charged particles found in the nucleus. Protons are one of the fundamental particles that make up the nucleus of an atom. They are positively charged particles, and their presence is crucial for defining the identity of an element.
Protons were discovered by Ernest Rutherford in 1919. He observed that when alpha particles were shot into nitrogen gas, hydrogen nuclei were produced. This led to the conclusion that hydrogen nuclei (which we now call protons) are a part of other elements’ nuclei.
- Charge: Each proton carries a positive charge, which is equal in magnitude but opposite in sign to the charge of an electron.
- Mass: The mass of a proton is approximately \(\displaystyle 1.6726 \times 10^{-27} \text{ kg}\), which is nearly 1836 times the mass of an electron. Protons reside in the nucleus of an atom, contributing to the overall positive charge and mass of the nucleus.
The number of protons in the nucleus of an atom determines the atomic number (Z), which defines the type of element. For example, an atom with one proton is hydrogen, while an atom with six protons is carbon. The number of protons in an atom influences the chemical behavior of the element, as it determines the number of electrons that can be held in the electron cloud, which in turn affects how the atom will bond with others.
Inside the nucleus, protons repel each other due to their like charges. However, this repulsion is overcome by the strong nuclear force, which binds protons and neutrons together in the nucleus. Protons contribute significantly to the mass of an atom. The atomic mass unit (u) is defined such that one proton (or one neutron) has a mass of approximately 1u.
Neutrons: Neutral particles that also reside in the nucleus. Neutrons are subatomic particles that, along with protons, form the nucleus of an atom. They play a critical role in the stability and mass of atoms but do not contribute to the electrical charge.
Neutrons were discovered by James Chadwick in 1932. He observed that when beryllium was bombarded with alpha particles, a new type of radiation was produced, which he concluded was made up of neutral particles—neutrons.
- Charge: Neutrons are electrically neutral, meaning they have no charge.
- Mass: The mass of a neutron is slightly greater than that of a proton, at about \(\displaystyle 1.675 \times 10^{-27} \text{ kg}\). Neutrons are located in the nucleus alongside protons.
Neutrons contribute to the stability of the nucleus. Without neutrons, the repulsive forces between the positively charged protons would cause the nucleus to fly apart. The number of neutrons can vary in atoms of the same element, leading to different isotopes. Isotopes have the same number of protons but different numbers of neutrons.
Neutrons are bound to protons through the strong nuclear force, which is much stronger than the electromagnetic force repelling the positively charged protons. Like protons, neutrons contribute significantly to the mass of an atom. They are measured in atomic mass units (u), with one neutron having a mass of approximately 1u.
Electrons: Negatively charged particles that orbit the nucleus. Electrons are subatomic particles with a negative charge, and they play a vital role in defining the chemical properties of an element.
Electrons were discovered by J.J. Thomson in 1897 during his experiments with cathode rays. He found that these rays were composed of previously unknown negatively charged particles, which he called electrons.
- Charge: Electrons carry a negative charge, which is equal in magnitude but opposite to the charge of a proton.
- Mass: Electrons have a very small mass of about \(\displaystyle 9.109 \times 10^{-31} \text{ kg}\), which is approximately 1/1836th the mass of a proton. Electrons are located in the space around the nucleus, within regions called electron shells or orbitals.
Electrons are responsible for the chemical reactions between atoms. The arrangement of electrons in the outermost shell, or valence shell, determines how an atom will interact with others. The flow of electrons is what we observe as electricity. In metals, for example, the outer electrons are free to move, allowing them to conduct electric current.
The behavior of electrons is governed by the principles of quantum mechanics. Unlike larger objects, electrons do not have a defined position and instead exist in states of probability, known as quantum states. Electrons occupy discrete energy levels, and they can move between these levels by absorbing or emitting energy, often in the form of light.
While the nucleus determines the mass of an atom, the electron cloud determines the size of the atom. When atoms gain or lose electrons, they become ions. This process is essential for the formation of compounds.
Nucleus Discovery
The nucleus is the central part of an atom, but it wasn’t always known to scientists. The discovery of the nucleus is a story of scientific inquiry and experimentation. Initially, atoms were thought to be indivisible and the smallest units of matter. This view changed with the discovery of subatomic particles.
The nucleus was identified as a result of experiments aimed at understanding the nature of electricity and matter. Scientists like J.J. Thomson and Ernest Rutherford were instrumental in these discoveries.
Thomson’s Experiments with Cathode Ray Tubes:
J.J. Thomson’s groundbreaking experiments in the late 19th century were pivotal in the discovery of the electron and led to the concept of the atomic nucleus.
A cathode ray tube (CRT) is a sealed glass tube from which most of the air has been evacuated. It contains two metal electrodes: the cathode (negative electrode) and the anode (positive electrode). When a high voltage is applied across these electrodes, a stream of particles known as cathode rays travels from the cathode to the anode.
Thomson used the CRT to show that cathode rays were composed of particles that had mass and a negative charge. He did this by applying electric and magnetic fields perpendicular to the path of the rays and observing how the rays were deflected. The deflection could only be explained if the rays were made up of particles that were much lighter than atoms and carried a negative charge.
Thomson’s work demonstrated that atoms were not the smallest units of matter and that they contained smaller, negatively charged particles – electrons. This was contrary to the prevailing belief at the time that atoms were indivisible. Thomson’s discovery of the electron was the first indication that an atom had structure and that there was something within it, which later turned out to be the nucleus.
Following his discovery, Thomson proposed the plum pudding model of the atom. In this model, the atom was envisioned as a sphere of positive charge with electrons (the ‘plums’) scattered throughout, like raisins in a pudding. This model set the stage for future experiments that would further refine our understanding of atomic structure.
Thomson’s experiments with cathode ray tubes were a fundamental step toward the modern understanding of the atom. They introduced the concept of subatomic particles and paved the way for the discovery of the nucleus.
Rutherford’s Gold Foil Experiment and Atomic Model:
In 1911, Ernest Rutherford designed an experiment that would revolutionize our understanding of the atom. The experiment involved firing a beam of alpha particles at a very thin sheet of gold foil.
Alpha particles These are positively charged particles with a mass about four times that of a hydrogen atom. Rutherford used a thin sheet of gold because it could be made just a few atoms thick. A fluorescent screen around the foil allowed the scientists to see where the alpha particles went after hitting the foil.
Before this experiment, the accepted model (Thomson’s plum pudding model) suggested that the atom was a diffuse cloud of positive charge with electrons embedded within it. According to this model, the alpha particles should have passed through the foil with little deflection. While most alpha particles passed through the foil, a small number were deflected at large angles, and some even bounced back toward the source.
The only explanation for such deflections was the presence of a small, dense, positively charged center in the atom. This center was named the nucleus. Rutherford proposed that the electrons orbit this nucleus, much like planets orbit the sun, with most of the atom being empty space.
This experiment was crucial because it provided the first evidence of a nucleus within the atom, leading to the nuclear model of the atom we use today. It showed that the atom has a tiny, dense core where most of its mass and positive charge are concentrated, surrounded by a cloud of electrons.
Neutrons:
Neutrons are subatomic particles found in the nucleus of an atom. They are unique because they carry no electric charge, which is why their discovery was more challenging than that of charged particles like protons and electrons. Before neutrons were discovered, scientists had noticed that the atomic masses of most elements were about twice their atomic numbers. This was puzzling because the known protons couldn’t account for all the mass.
In 1932, James Chadwick conducted a series of experiments that led to the discovery of the neutron. He bombarded beryllium with alpha particles and observed that a new type of radiation was emitted, which did not consist of charged particles like protons or electrons.
Chadwick’s experiments showed that this radiation could penetrate materials that stopped alpha and beta particles, suggesting that the particles had no charge. By measuring the energy of the recoiling particles, Chadwick concluded that these neutral particles had a similar mass to protons.
The discovery of neutrons explained why atoms were heavier than the total mass of their protons and electrons. It also led to the understanding that neutrons, along with protons, make up the nucleus of an atom. It completed the picture of the nucleus as composed of protons and neutrons, and it opened up new areas of research, including nuclear fission and the development of nuclear energy.
Composition of a Nucleus
The nucleus consists of protons, which are positively charged particles, and neutrons, which have no charge. These particles are collectively known as nucleons. Protons and neutrons are held together by a strong force, known as the strong nuclear force, which is responsible for the nucleus’s stability. This force overcomes the electrostatic repulsion between the positively charged protons.
The nuclear forces are the strongest forces observed within the context of the nucleus. They operate at very short ranges and are responsible for binding protons and neutrons into a compact nucleus despite the repulsive forces. Inside the nucleus, protons and neutrons are arranged in an incredibly dense configuration. Despite occupying a tiny volume within the atom, the nucleus contains almost all of the atom’s mass.
Characteristics of Nucleus:
It is positively charged due to protons and contains nearly all the atom’s mass. The nucleus is the central core of an atom and has several defining characteristics:
- Positive Charge: The nucleus carries a positive charge due to the presence of protons. Each proton has a positive charge, and it is the total number of protons that gives the nucleus its overall positive charge.
- Contains Nearly All the Atom’s Mass: Although the nucleus is very small compared to the entire atom, it contains nearly all of the atom’s mass. This is because protons and neutrons, which make up the nucleus, are much heavier than electrons.
- Dense: The nucleus is extremely dense. If we could create a cube that is 1 centimeter on each side from nuclear material, it would weigh about 100 million tons. This is because protons and neutrons are packed closely together in the nucleus.
- Holds Most of the Atom’s Mass: Protons and neutrons, which are roughly equal in mass, account for nearly all of the mass of the atom. Electrons contribute very little to the overall mass.
- Center of the Atom: The nucleus is located at the center of the atom. It is orbited by electrons, which are attracted to the nucleus because of its positive charge.
- Nuclear Forces: The nucleus stays together due to the strong nuclear force, which is one of the fundamental forces in nature. This force acts between nucleons (protons and neutrons) and is much stronger than the electromagnetic force that would otherwise cause the protons to repel each other.
Mass of a Nucleus:
The mass of a nucleus is a fundamental concept in physics, particularly in understanding atomic structure and nuclear reactions. The nucleus is composed of protons and neutrons, collectively known as nucleons. These particles are relatively heavy, especially when compared to electrons, and they account for almost all the mass of an atom.
The mass of a nucleus is measured in atomic mass units (u). One atomic mass unit is defined as one-twelfth the mass of a carbon-12 atom. The actual mass of a nucleus is slightly less than the sum of the masses of its protons and neutrons. This difference is known as the mass defect. It occurs because some mass is converted into energy that binds the nucleus together, according to Einstein’s equation (E = mc2).
The total number of protons and neutrons in a nucleus is called the atomic mass number (A). It gives us a close approximation of the nucleus’s mass.
Size of Nucleus:
The nucleus is incredibly small compared to the overall size of the atom. If we compare the size of the nucleus to the size of the atom, the nucleus is much smaller. The radius of a nucleus is about 10,000 times smaller than the radius of an atom.
Because of this vast difference in size, the volume of the nucleus is about 10-12 times the volume of the atom. This means that the atom is mostly empty space. The size of the nucleus was first estimated through Rutherford’s gold foil experiment. By observing the scattering of alpha particles, scientists concluded that the nucleus occupies a very small portion of the atom’s volume.
The typical nuclear radius can be estimated using the formula
\(\displaystyle R = R_0 A^{1/3} \)
where (R0) is a constant approximately equal to \(\displaystyle 1.2 \times 10^{-15} \text{m}\) and (A) is the atomic mass number, which is the total number of protons and neutrons in the nucleus.
Atomic Number
The atomic number, denoted as (Z), is a fundamental property of an atom that defines the element to which the atom belongs.
” The atomic number is the total number of protons in the nucleus of an atom.”

Since protons carry a positive charge, the atomic number also represents the positive charge of the nucleus. Every element on the periodic table is uniquely identified by its atomic number. For instance, hydrogen has an atomic number of 1, meaning every hydrogen atom has one proton in its nucleus. Oxygen has an atomic number of 8, which means an oxygen atom has eight protons.
In a neutral atom, where the overall charge is zero, the number of electrons equals the atomic number. This balance between protons and electrons is what makes the atom electrically neutral. The atomic number determines the chemical properties of an element because it defines the electron configuration.
The arrangement of electrons around the nucleus, especially the electrons in the outermost shell, dictates how an atom will interact with other atoms. The elements in the periodic table are arranged in order of increasing atomic number. This arrangement reflects the repeating pattern of chemical properties known as the periodic law.
Atomic Mass
“The atomic mass (A) is the total number of protons and neutrons in the nucleus.”
The atomic mass of an element is a fundamental concept in chemistry and physics. It refers to the mass of an atom, typically expressed in atomic mass units (u). Atomic mass is the weighted average mass of the atoms in a naturally occurring sample of an element. It reflects the mass of both the protons and neutrons in the nucleus, as the mass of electrons is negligible due to their much smaller size.
Atomic Mass Unit (u) is a standard unit of mass that quantifies atomic mass. One atomic mass unit is defined as one-twelfth the mass of a carbon-12 atom, which has six protons and six neutrons.
Elements exist as isotopes, which are atoms with the same number of protons but different numbers of neutrons. The atomic mass is a weighted average of all the isotopes of that element, taking into account their relative abundance on Earth.
Example: For carbon, the atomic mass is 12.011 u. This value accounts for the natural abundance of Carbon-12 (with 6 protons and 6 neutrons) and other isotopes like Carbon-13 and Carbon-14.
The atomic mass allows chemists to count atoms by weighing them. Using the concept of moles, which is a unit of measurement for the amount of substance, scientists can relate the mass of an element to the number of atoms it contains.
Types of Nuclei
The nucleus of an atom can vary in its composition, leading to different types of nuclei. Here are the main categories:
Isotopes:
Isotopes are variants of a particular chemical element that have the same number of protons (and hence the same atomic number) but different numbers of neutrons. This means that while they are the same element, they have different mass numbers.
All isotopes of an element share the same chemical properties because they have the same number of electrons. However, their physical properties may differ due to the difference in mass. Most elements exist as a mixture of isotopes. For example, carbon naturally occurs mainly as Carbon-12 and Carbon-13, with a trace amount of Carbon-14.
Isotopes are often denoted by the element’s symbol followed by the mass number. For instance, the isotopes of hydrogen are protium (1H), deuterium (2H), and tritium (3H), where the superscript indicates the mass number (the total number of protons and neutrons).
Examples:
- Carbon Isotopes: Carbon-12 has 6 protons and 6 neutrons, Carbon-13 has 6 protons and 7 neutrons, and Carbon-14 has 6 protons and 8 neutrons. Carbon-14 is radioactive and is used in radiocarbon dating.
- Hydrogen Isotopes: The most common isotope of hydrogen, protium, has no neutrons, deuterium has one neutron, and tritium has two neutrons. Tritium is also radioactive.
Some isotopes are unstable and radioactive. These isotopes can be used in medical treatments, as tracers in biological and chemical research, and in dating ancient objects. Stable isotopes do not undergo radioactive decay and are used in various scientific applications, including climate studies and forensic analysis.
Isobars:
Isobars are nuclei that have the same mass number but different atomic numbers. This means they have the same total number of protons and neutrons, but the distribution between these two types of nucleons is different.
The mass number (A) is the sum of the number of protons (Z) and the number of neutrons (N) in the nucleus. Isobars have the same mass number. Even though isobars have the same mass number, they are different chemical elements because they have different numbers of protons.
Example: An example of isobars would be Argon-40 (18 protons and 22 neutrons) and Calcium-40 (20 protons and 20 neutrons). Both have a mass number of 40 but differ in their atomic numbers.
Isobars can have different stabilities. Some may be stable, while others may be radioactive. Understanding isobars is important in nuclear chemistry and physics, as different isobars can participate in or result from nuclear reactions.
Isotones:
Isotones are nuclei that have the same number of neutrons but different numbers of protons. This means that while they have different atomic numbers and potentially belong to different elements, they share a common neutron count.
The defining characteristic of isotones is their neutron number. It remains constant across isotones. Isotones are not the same element, as their number of protons (and hence their atomic number) varies. This difference in proton number makes them distinct elements on the periodic table.
Example: An example of isotones would be Carbon-14 (6 protons and 8 neutrons) and Nitrogen-15 (7 protons and 8 neutrons). Both have 8 neutrons but differ in their proton count.
Isotones can provide insights into nuclear stability. Nuclei with the same number of neutrons can have different stability levels based on their proton count. Isotones are important in the study of nuclear reactions, especially in understanding how changes in neutron number affect nuclear properties.
Mirror Nuclei:
Mirror nuclei are pairs of nuclei where the number of protons in one nucleus is equal to the number of neutrons in the other, and vice versa. These pairs of nuclei have the same mass number but opposite proton and neutron numbers. Although the proton and neutron numbers are reversed, mirror nuclei have the same mass number because the sum of protons and neutrons remains constant.
In mirror nuclei, the number of protons in one nucleus is the same as the number of neutrons in its mirror counterpart. Mirror nuclei pairs have equal spins and the same parity, which means they share similar nuclear properties.
Examples:
- Carbon-14 and Oxygen-14: Carbon-14 has 6 protons and 8 neutrons, while Oxygen-14 has 8 protons and 6 neutrons. They are mirror nuclei because the proton number of one is the neutron number of the other.
- Nitrogen-15 and Oxygen-15: Nitrogen-15 has 7 protons and 8 neutrons, and Oxygen-15 has 8 protons and 7 neutrons. They also form a pair of mirror nuclei.
Isomer Nuclei:
Isomer nuclei are nuclei that have the same atomic number and mass number but exist in different energy states. This means they are the same element, with the same number of protons and neutrons, but their internal arrangements are different, leading to different energy levels.
Isomer nuclei belong to the same element because they have the same number of protons. Despite having the same atomic and mass numbers, isomer nuclei have different nuclear energy states. This can affect their nuclear properties, such as their stability and the way they decay. Some isomer nuclei are in metastable states, meaning they are not in the lowest possible energy state and can release energy as they transition to a more stable state.
Example: A common example of an isomer nucleus used in medical imaging is Technetium-99m. It has the same number of protons and neutrons as Technetium-99 but is in a higher energy state. It emits gamma rays as it decays to the lower energy state of Technetium-99.
Also Read: Bohr Model of Hydrogen Atom
Solved Examples
Problem 1: A nucleus of Helium-4 (\(\displaystyle {}_{2}^{4}He\)) has an atomic mass of 4.0026 u. The mass of a proton is 1.0073 u and the mass of a neutron is 1.0087 u. Calculate the mass defect and the binding energy of the Helium-4 nucleus. (1 u = 931 MeV/c²)
Solution: First, calculate the mass of the individual nucleons:
\(\displaystyle\text{Mass of 2 protons} = 2 \times 1.0073 \, \text{u} = 2.0146 \, \text{u}\)
\(\displaystyle\text{Mass of 2 neutrons} = 2 \times 1.0087 \, \text{u} = 2.0174 \, \text{u}\)
Total mass of nucleons = 2.0146 u + 2.0174 u = 4.0320 u
The mass defect (∆m) is:
\(\displaystyle\Delta m = \text{Total mass of nucleons} – \text{Atomic mass of } ^4_2\text{He}\)
\(\displaystyle\Delta m = 4.0320 \, \text{u} – 4.0026 \, \text{u} = 0.0294 \, \text{u}\)
The binding energy (BE) is:
\(\displaystyle\text{BE} = \Delta m \times 931 \, \text{MeV/u}\)
\(\displaystyle\text{BE} = 0.0294 \times 931 = 27.38 \, \text{MeV}\)
The mass defect of the Helium-4 nucleus is 0.0294 u, and the binding energy is 27.38 MeV.
Problem 2: An atom has 15 protons and 16 neutrons. Identify the element and determine its atomic number and mass number.
Solution: The atomic number (Z) is the number of protons:
Z = 15 , The mass number (A) is the sum of protons and neutrons:
A = Z + number of neutrons = 15 + 16 = 31
Using the periodic table, the element with atomic number 15 is Phosphorus (P).
The element is Phosphorus (P) with an atomic number of 15 and a mass number of 31.
Problem 3: In Rutherford’s gold foil experiment, alpha particles were directed at a thin gold foil. If the thickness of the gold foil is 0.01 mm and the number of gold atoms per cubic meter is (\(\displaystyle 5.9 \times 10^{28}\)), calculate the number of gold atoms in the foil per square meter.
Solution: The thickness of the gold foil (t) in meters:
\(\displaystyle t = 0.01 \, \text{mm} = 0.01 \times 10^{-3} \, \text{m} = 10^{-5} \, \text{m}\)
The number of gold atoms per cubic meter is given as:
\(\displaystyle N = 5.9 \times 10^{28} \, \text{atoms/m}^3\)
The number of atoms in the foil per square meter (n) is:
\(\displaystyle n = N \times t\)
\(\displaystyle n = 5.9 \times 10^{28} \times 10^{-5} = 5.9 \times 10^{23} \, \text{atoms/m}^2\)
The number of gold atoms in the foil per square meter is (\(\displaystyle 5.9 \times 10^{23} \)).
Problem 4: Chlorine has two naturally occurring isotopes: (35Cl) with an abundance of 75.78% and 37Cl with an abundance of 24.22%. Calculate the average atomic mass of chlorine.
Solution: The atomic masses of 35Cl and 37Cl are approximately 34.969 u and 36.966 u, respectively.
The average atomic mass (M) is:
\(\displaystyle M = \left( \frac{75.78}{100} \times 34.969 \right) + \left( \frac{24.22}{100} \times 36.966 \right)\)
Calculate each term:
\(\displaystyle M = 0.7578 \times 34.969 + 0.2422 \times 36.966\)
\(\displaystyle M = 26.51 + 8.95 = 35.46 \, \text{u}\)
The average atomic mass of chlorine is 35.46 u.
Problem 5: Determine the number of protons, neutrons, and electrons in (\(\displaystyle {}_{{26}}^{{56}}F{{e}^{{3+}}}\)).
Solution: For (\(\displaystyle {}_{{26}}^{{56}}F{{e}^{{3+}}}\)):
The number of protons (Z) is equal to the atomic number: ( Z = 26 ). The mass number (A) is 56.
The number of neutrons is:
Neutrons = A – Z = 56 – 26 = 30
The number of electrons in a neutral Fe atom is 26. Since it is (3+) ion, it has lost 3 electrons:
Electrons= 26 – 3 = 23
The ion (\(\displaystyle {}_{{26}}^{{56}}F{{e}^{{3+}}}\)) has 26 protons, 30 neutrons, and 23 electrons.
Problem 6: Given the binding energies of (\(\displaystyle {}_{6}^{{12}}C\)) and (\(\displaystyle {}_{{26}}^{{56}}Fe\)) are 92 MeV and 492 MeV, respectively, calculate the binding energy per nucleon for both nuclei and determine which is more stable.
Solution: For (\(\displaystyle {}_{6}^{{12}}C\)): Number of nucleons = 12
Binding energy per nucleon = (\(\displaystyle\frac{92 \, \text{MeV}}{12}\))
\(\displaystyle\frac{92}{12} = 7.67 \, \text{MeV/nucleon}\)
For (\(\displaystyle {}_{{26}}^{{56}}Fe\)): Number of nucleons = 56
Binding energy per nucleon = (\(\displaystyle\frac{492 \, \text{MeV}}{56}\))
\(\displaystyle\frac{492}{56} = 8.79 \, \text{MeV/nucleon}\)
The binding energy per nucleon for (\(\displaystyle {}_{6}^{{12}}C\)) is 7.67 MeV/nucleon, and for (\(\displaystyle {}_{{26}}^{{56}}Fe\)) is 8.79 MeV/nucleon. The nucleus (\(\displaystyle {}_{{26}}^{{56}}Fe\)) is more stable due to its higher binding energy per nucleon.
FAQs
What is the structure of an atom and how are its components arranged?
An atom consists of a central nucleus surrounded by electrons. The nucleus contains protons, which are positively charged, and neutral neutrons. Electrons, which are negatively charged, orbit the nucleus in various energy levels or shells. The protons and neutrons are tightly bound in the nucleus, while the electrons move in defined regions around the nucleus.
How was the nucleus of an atom discovered?
The nucleus was discovered by Ernest Rutherford in 1911 through his famous gold foil experiment. Rutherford directed alpha particles at a thin sheet of gold foil and observed their scattering patterns. He found that most particles passed through the foil, but some were deflected at large angles, indicating a small, dense, positively charged center within the atom, which he called the nucleus.
What is the composition of a nucleus?
The nucleus of an atom is composed of protons and neutrons, collectively known as nucleons. Protons carry a positive charge, while neutrons have no charge. The number of protons in the nucleus determines the atomic number and the element’s identity, while the combined number of protons and neutrons gives the atomic mass number.
What is an atomic number and why is it important?
The atomic number is the number of protons in the nucleus of an atom. It is important because it uniquely identifies an element and determines its position in the periodic table. The atomic number also defines the chemical properties of the element, as it dictates the number of electrons in a neutral atom, which in turn influences how the element interacts with others.
What is atomic mass and how is it different from atomic number?
Atomic mass, also known as atomic mass number or nucleon number, is the total number of protons and neutrons in the nucleus of an atom. It is different from the atomic number, which only counts the protons. While the atomic number identifies the element, the atomic mass gives information about the specific isotope of that element, as different isotopes have different numbers of neutrons.
How do electrons contribute to the structure and properties of an atom?
Electrons contribute to the structure of an atom by occupying various energy levels or shells around the nucleus. They play a crucial role in determining the atom’s chemical properties and behavior. Electrons are involved in forming chemical bonds, conducting electricity, and participating in reactions. The arrangement of electrons in an atom’s energy levels determines its reactivity and the types of bonds it can form.
How can you determine the number of neutrons in an atom?
The number of neutrons in an atom can be determined by subtracting the atomic number from the atomic mass number. The atomic number gives the number of protons, and the atomic mass number represents the total number of protons and neutrons. Thus, by knowing these two values, the number of neutrons can be calculated. For example, if an atom has an atomic mass number of 23 and an atomic number of 11, it has 12 neutrons.