The story of electric charge begins in ancient times. The Greeks were among the first to discover electrical phenomena. Around 600 BC, a Greek philosopher named Thales of Miletus observed that rubbing amber with fur would cause it to attract small objects like dust and feathers. This was the earliest known study of static electricity, which they believed was a magnetism specific to amber.
Fast forward to the 17th and 18th centuries, when scientists began to understand electricity more scientifically, Sir William Gilbert, an English physician, coined the term “electric” to describe materials like amber that attracted other objects after being rubbed. He is often credited with being the father of electrical studies.
In 1729, Stephen Gray distinguished between conductors and insulators, showing that some materials could transfer charge while others could not. This led to further experiments and discoveries by scientists like Charles du Fay, who found that there were two kinds of electric charge, which he called “vitreous” and “resinous,” now known as positive and negative.
Benjamin Franklin, the American polymath, conducted his famous kite experiment in 1752, proving that lightning was a form of electricity. He also proposed the single fluid theory of electricity and introduced terms like “battery,” “conductor,” and “electrician.”
The 19th century saw significant advancements with the work of Michael Faraday, who studied electromagnetic induction, and James Clerk Maxwell, who formulated the classical theory of electromagnetic radiation, bringing together for the first time electricity, magnetism, and light as different manifestations of the same phenomenon.
In the 20th century, the quantum theory of charge was developed. Scientists like J.J. Thomson discovered the electron in 1897, which led to the understanding that electric charge is carried by particles. Later, the discovery of the proton and neutron completed the picture of how atoms are constructed and how they carry charge.
What is an Electric Charge?
An electric charge is a fundamental property of matter that is observed in the form of either a positive or negative charge. It is carried by the subatomic particles of matter: protons have a positive charge, and electrons have a negative charge. When matter experiences a force within an electric or magnetic field, it is due to the presence of an electric charge.
Electric charge is not a vector quantity. Unlike vector quantities, which have both magnitude and direction, electric charge only has magnitude and is considered a scalar quantity. It does not follow the laws of vector addition like the triangle law or parallelogram law.
The unit of electric charge is the coulomb (C), which is the standard unit of measure in the International System of Units (SI). One coulomb is defined as the amount of charge transferred by a current of one ampere flowing for one second.
Imagine you have two magnets. When you bring the opposite poles close, they attract each other, but when you try to push the same poles together, they repel. The electric charge works similarly but with particles instead of magnets.
An electric charge is a fundamental property of particles that make up matter, like protons and electrons. It’s what causes these particles to experience a force when they’re near other charged particles or in an electric or magnetic field.
- Protons are like tiny balls with a positive (+) sign painted on them.
- Electrons are like tiny balls with a negative (-) sign painted on them.
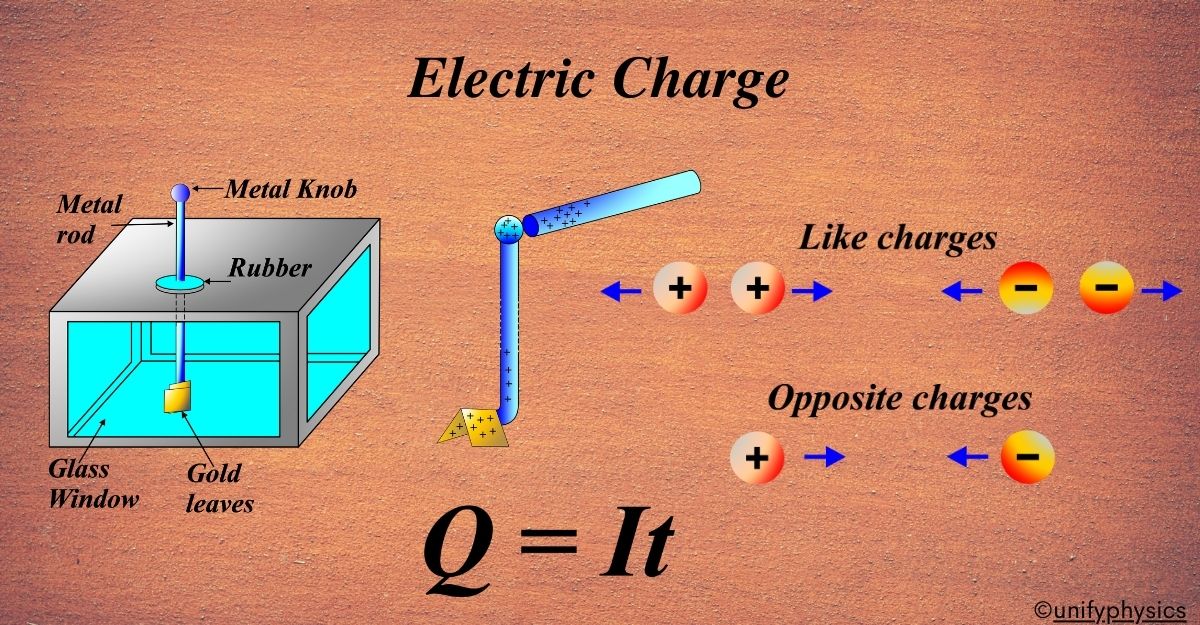
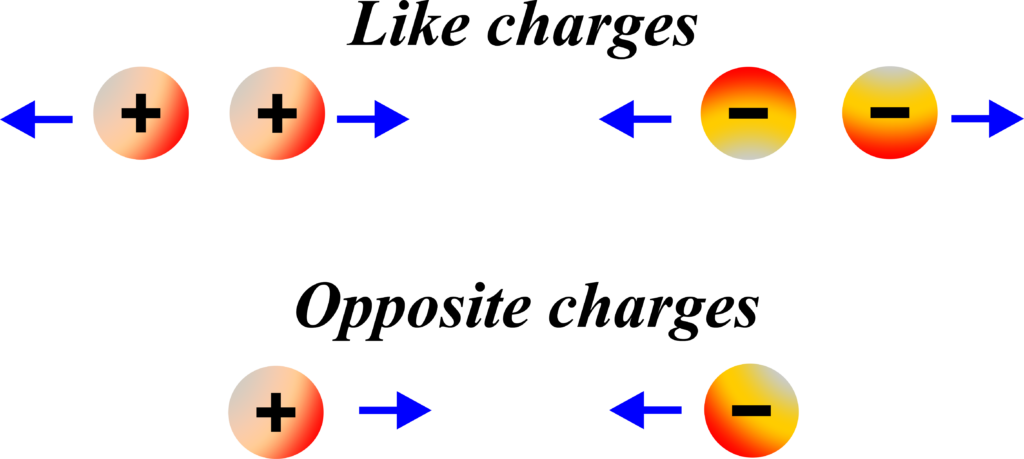
When a proton and an electron are near each other, they’re attracted because they have opposite charges, just like the opposite poles of a magnet. But if you have two protons or two electrons close to each other, they’ll repel because they have the same charge.
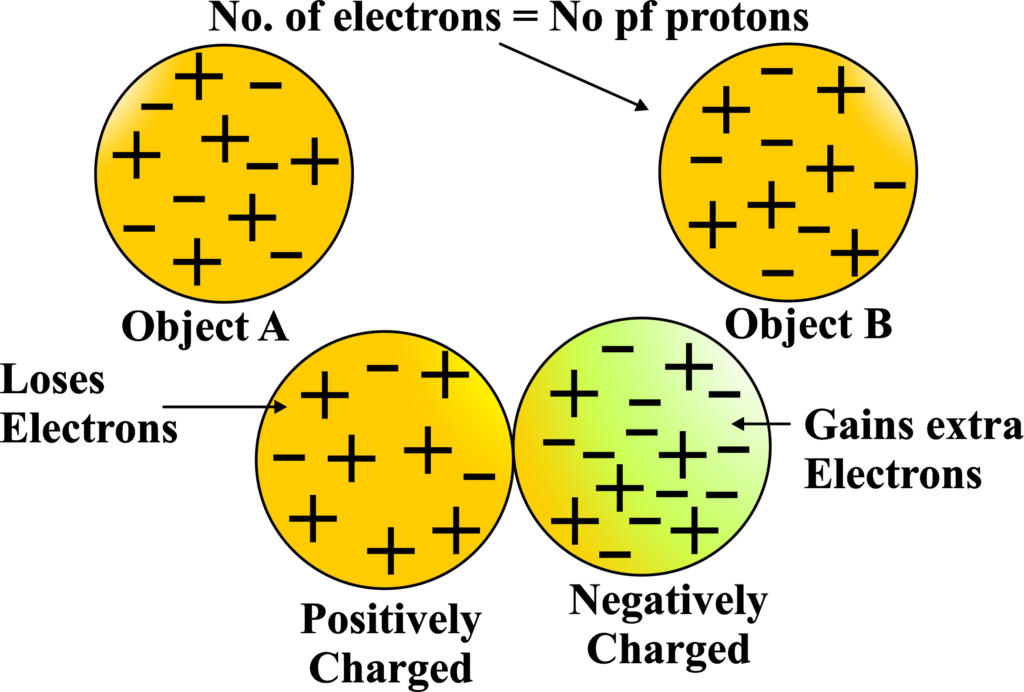
In most objects, the number of protons and electrons is balanced, so they cancel each other out, and the object doesn’t have an overall charge. But if an object has more electrons than protons, it will have a negative charge, and if it has more protons than electrons, it will have a positive charge.
Types of Electric Charge
Electric charge is one of the most fundamental concepts in physics, and it comes in two main types: positive and negative. Here’s a detailed look at each type:
Positive Charge:
- Protons are the bearers of positive charge.
- They reside in the nucleus of an atom and are denoted by the symbol +.
- When an object has more protons than electrons, it is considered to have a positive charge.
- Positively charged objects will attract negatively charged objects and repel other positively charged objects.
Negative Charge:
- Electrons carry a negative charge.
- They orbit around the nucleus of an atom and are represented by the symbol –.
- An object with more electrons than protons has a negative charge.
- Negatively charged objects will attract positively charged objects and repel other negatively charged objects.
Neutral Charge:
- When the number of protons and electrons in an object is equal, the charges cancel each other out, resulting in a neutral charge.
- Neutral objects do not exhibit attraction or repulsion towards other charged objects unless induced by an external electric field.
Measuring Electric Charge
When we talk about measuring electric charge, we’re referring to determining the quantity of charge an object has. This is a fundamental concept in physics, especially in the study of electrostatics.
The standard unit of electric charge in the International System of Units (SI) is the coulomb (C). It’s named after Charles-Augustin de Coulomb, a French physicist who made significant contributions to the theory of electrostatics.
One coulomb is defined as the amount of charge transferred by a constant current of one ampere flowing for one second. Mathematically, this is represented as:
\(\displaystyle Q = I \cdot t \)
- (Q) is the electric charge in coulombs.
- (I) is the electric current in amperes.
- (t) is the time in seconds during which the current flows.
In practice, measuring the charge directly can be challenging because it’s not something we can see or touch. Instead, we often measure the effects of the charge, such as the force between charged objects or the current produced when charges move.
Gold-Leaf Electroscope Experiment:
A gold-leaf electroscope is a device used to detect the presence and magnitude of electric charge on a body. It consists of a metal rod with a knob at the top, from which two thin gold leaves hang down. The metal rod is inserted into a glass or plastic container, known as the electroscope case, which is typically made of an insulating material.
A gold-leaf electroscope consists of:
- A vertical metal rod from which two thin gold leaves are suspended.
- A metal disc at the top of the rod is used to introduce charge to the electroscope.
- The rod and leaves are enclosed in a glass jar to prevent air currents from moving the leaves.
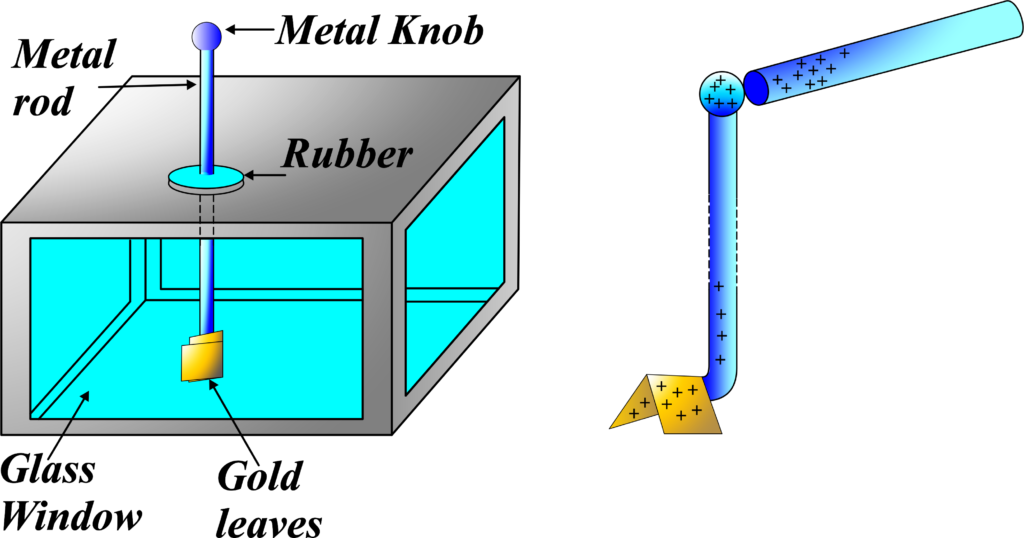
Principle
The gold-leaf electroscope works on the principle that like charges repel each other. When a charge is introduced to the metal disc, it distributes along the rod and to the leaves. Since both leaves acquire the same type of charge, they repel each other and diverge.
Steps of the Experiment:
- Starting Condition: Begin with the electroscope uncharged, with the gold leaves hanging down.
- Introducing Charge: Bring a charged object (like a rubbed plastic rod) close to the metal disc at the top of the electroscope.
- Observation: If the object is charged, the leaves will repel each other and diverge. The greater the charge, the wider the leaves will spread apart.
- Testing Charge Polarity: To determine the polarity of the charge, you can bring a known charged object near the electroscope. If the leaves diverge further, the charges are the same. If they collapse, the charges are the opposite.
While the electroscope does not provide a quantitative measure of charge, it does give a qualitative indication. The degree of divergence of the leaves can give an approximate idea of the amount of charge present.
Applications
- Detecting Charge: To check if an object is charged, touch it to the metal disc. If the leaves diverge, the object is charged.
- Identifying Charge Type: By using a known charged object, you can determine whether an unknown charge is positive or negative based on the behavior of the leaves.
- Demonstrating Induction: You can demonstrate charging by induction by bringing a charged object near the electroscope and grounding the electroscope momentarily.
Safety Precautions: Ensure the glass jar is clean and dry to prevent leakage of charge. Handle the gold leaves with care as they are very delicate and can tear easily.
This experiment is a classic method to demonstrate the presence and nature of electric charge, and it provides a visual way for students to understand the fundamental concepts of electrostatics.
For example, if we have a known current flowing for a certain amount of time, we can calculate the total charge transferred using the formula above. Alternatively, we can use instruments like electrometers or charge sensors to measure the potential difference or electric field, which can then be related to the charge.
It’s also important to remember that charge is quantized, meaning it comes in discrete packets. The smallest unit of charge is the charge of one electron, approximately (-1.6× 10-19) C. All observable charges are whole-number multiples of this elementary charge.
Properties of Electric Charge
Additivity:
The concept of additivity is one of the fundamental properties of electric charge. It’s a principle that allows us to understand how charges combine when multiple charged objects are brought together.
Additivity means that the total electric charge of a system is the algebraic sum of all the individual charges present within that system. This property is crucial because it helps us calculate the net charge in any given scenario.
Imagine you have a collection of charged particles, some with positive charges and some with negative ones. To find the total charge, you simply add up all the charges, taking into account their signs:
- Positive charges are added normally.
- Negative charges are subtracted (since they are essentially positive values with a negative sign).
Example: Let’s say we have three objects with the following charges:
- Object A has a charge of +3 coulombs (C).
- Object B has a charge of -2 C.
- Object C has a charge of +1 C.
To find the total charge, we add them up:
\(\displaystyle Total\ Charge = (+3\ C) + (-2\ C) + (+1\ C) = +2\ C \)
This means the system has a net charge of +2 C.
Points to Remember:
- Charges are scalar quantities, which means they have magnitude but no direction.
- The sign of the charge (positive or negative) is crucial when applying the principle of additivity.
- The additivity of charge is consistent with the conservation of charge, which states that the total charge in an isolated system remains constant.
Conservation:
The conservation of electric charge is a fundamental principle in physics, particularly in the field of electromagnetism. It states that the total electric charge in an isolated system remains constant over time. This means that charge can neither be created nor destroyed; it can only be transferred from one object to another.
To understand this concept, let’s consider a simple analogy. Imagine you have a set of colored balls in a closed box. You can move the balls around inside the box, but you can’t add new ones or take any out. The total number of balls in the box remains the same, no matter how they are arranged. Similarly, in an isolated system, the total amount of charge stays constant, even though individual charges may move from one place to another.
Examples:
- Charging by Friction: When you rub a balloon on your hair, electrons are transferred from your hair to the balloon. Your hair becomes positively charged (losing electrons), and the balloon becomes negatively charged (gaining electrons). The total charge before and after rubbing remains the same; it’s just redistributed.
- Chemical Reactions: In chemical reactions, atoms may gain or lose electrons during bond formation or breaking. However, the total charge of the reactants and the products remains the same.
Conservation of Charge:
- Electric Circuits: In an electric circuit, electrons flow from the negative terminal to the positive terminal of a battery. The total charge within the circuit doesn’t change; electrons are just moving through the circuit.
- Particle Physics: In particle physics, when particles are created or annihilated, the total charge before and after the event remains the same. For example, when an electron and a positron (its antiparticle) annihilate, they produce neutral photons, but the total charge is conserved.
The conservation of charge is important because it’s a law that helps us predict the behavior of charged particles in various scenarios. It’s a rule that nature seems to follow without exception, and it’s a cornerstone of our understanding of physical laws.
Quantization:
The quantization of electric charge is a fundamental principle that tells us that all free charges are an integral multiple of a basic unit of charge, denoted by (e). This means that the charge on any object is always a whole number multiple of this elementary charge.
To understand quantization, think of change as something that cannot be divided indefinitely. Just like you can’t have half a pencil as the smallest unit of pencils, you can’t have a fraction of an elementary charge as a charge on particles. It’s either one elementary charge, two, three, or so on, but never 1.5 or 2.7, and so on.
The elementary charge (e) is the smallest amount of charge that exists and is carried by subatomic particles like protons and electrons. The value of (e) is approximately (1.6×10-19) coulombs (C).
The charge (q) on any object can be expressed as:
\(\displaystyle q = n \times e \)
- (n) is an integer (which can be zero, positive, or negative).
- (e) is the elementary charge.
Examples:
- An electron has a charge of (-e) (negative elementary charge).
- A proton has a charge of (+e) (positive elementary charge).
- If an object has 5 excess electrons, its charge would be (-5e) or (-8 × 10-19) C.
Quantization of charge is important because it explains why we only observe certain specific amounts of charge in nature. It’s also crucial for the stability of atoms and the formation of matter as we know it.
Coulomb’s Law
Coulomb’s Law states that the force between two point charges is directly proportional to the product of their charges and inversely proportional to the square of the distance between them.
Coulomb’s Law is a fundamental principle in physics that describes the force between two point charges. It was formulated by the French physicist Charles-Augustin de Coulomb in 1785.
Coulomb’s Law states that the magnitude of the electrostatic force (F) between two point charges is directly proportional to the product of the magnitudes of the charges (q1) and (q2 ) and inversely proportional to the square of the distance (r) between them. The law can be expressed with the following formula:
\(\displaystyle F = k \frac{|q_1 \cdot q_2|}{r^2} \)
- (F) is the electrostatic force between the charges.
- (q1) and (q2) are the amounts of the charges.
- (r) is the distance between the centers of the two charges.
- (k) is Coulomb’s constant (8.9875×109 ) N·m²/C² in vacuum).
The force is attractive if the charges have opposite signs and repulsive if the charges have the same sign. It acts along the line joining the centers of the two charges.
In vector form, Coulomb’s Law takes into account the direction of the force and can be written as:
\(\displaystyle \vec{F}{12} = k \frac{q_1 \cdot q_2}{r^2} \hat{r}{12} \)
- \(\displaystyle \vec{F}_{12} \) is the force on charge (q1) due to (q2).
- \(\displaystyle \hat{r}_{12} \) is the unit vector from (q1) to (q2).
- Coulomb’s Law applies to point charges or spherically symmetric charge distributions.
- The law is valid in an inverse-square form, which means that as the distance between charges doubles, the force reduces to a quarter of its original value.
- The medium between the charges affects the force. In a medium other than a vacuum, the force is reduced by the dielectric constant of the medium.
Coulomb’s Law is used to calculate the force between two charged objects, which is essential in understanding concepts like electric fields, potentials, and the behavior of charges in various materials.
Transfer and Separation of Charge
Charges can be transferred from one object to another, often by direct contact or through a conducting path. Separation of charge can occur in various ways, such as by induction or polarization.
Transfer of Charge: The transfer of charge refers to the movement of electric charge from one object to another. This can occur through various methods, such as conduction, induction, and friction.
- Conduction: When a charged object comes into direct contact with another object, charges can move from one to the other. For example, if a negatively charged rod touches a neutral metal sphere, electrons will transfer to the sphere, making it negatively charged.
- Induction: A charged object brought near a neutral conductor can cause a redistribution of charges within the conductor without direct contact. Grounding the conductor during this process can result in a permanent transfer of charge.
- Friction: Rubbing two different materials together can cause electrons to transfer from one material to the other, resulting in one object becoming positively charged and the other negatively charged.
Separation of Charge: Separation of charge occurs within an object when there is a redistribution of charge due to an external influence, such as an electric field.
- Polarization: In insulators, an external electric field can cause a slight shift of charges, leading to a separation of positive and negative charges within the material. This creates a dipole effect, where one side of the object becomes slightly more positive and the other side slightly more negative.
- Induced Dipoles: In conductors, an external electric field can cause free electrons to move, creating regions of positive and negative charge within the object.
In both the transfer and separation of charge, the total charge remains conserved. This means that the total amount of charge before and after the process is the same. Charge is neither created nor destroyed; it is simply moved from one place to another or redistributed within an object.
Examples:
Static Electricity: When you walk across a carpet and then touch a metal doorknob, you might feel a shock. This is due to the transfer of charge that has built up on your body from the friction with the carpet.
Lightning: During a storm, charges separate within clouds, leading to a buildup of electrical energy. The rapid transfer of charge from the cloud to the ground or between clouds results in lightning.
Methods of Charging
Friction:
Charging by friction is one of the oldest known methods of charging objects. It occurs when two different materials are rubbed together, causing electrons to be transferred from one material to the other.
When two objects are rubbed against each other, they experience friction. This frictional force can cause electrons, which are the negatively charged particles of atoms, to move from one object to the other.
- The object that loses electrons will end up with a positive charge because it has more protons (which are positively charged) than electrons.
- Conversely, the object that gains electrons will have a negative charge.
Different materials have different tendencies to either gain or lose electrons. This is known as the triboelectric effect. Some materials, like glass, tend to lose electrons, while others, like rubber, tend to gain them.
Examples: When a glass rod is rubbed with silk, the glass rod tends to lose electrons and becomes positively charged, while the silk gains those electrons and becomes negatively charged.
Similarly, when a balloon is rubbed against hair, the balloon can gain electrons from the hair, leaving the hair positively charged and the balloon negatively charged.
It’s important to note that the total charge is conserved during this process. The amount of negative charge gained by one object is equal to the positive charge gained by the other object.
Factors Affecting Charging by Friction:
- Type of Material: Some materials are more prone to gaining or losing electrons.
- Surface Area: More area in contact can mean more electrons transferred.
- Roughness: Rougher surfaces can increase the friction and thus the transfer of electrons.
- Pressure: Applying more pressure can increase the contact between surfaces, leading to more electron transfer.
In summary, we can say that charging by friction is a fundamental electrostatic phenomenon where the transfer of electrons due to frictional contact results in one object becoming positively charged and the other negatively charged.
Conduction:
Charging by conduction, also known as charging by contact, is a process where a charged object is used to charge another object without transferring any matter between them. This method involves direct physical contact between the charged object and the neutral object.
When a charged conductor comes into contact with a neutral conductor, charges are transferred between them until both objects reach the same electrical potential. This means that the excess charge on the charged object will spread out over both objects.
- If the initially charged object is positively charged, it will transfer some of its positive charge to the neutral object. As a result, both objects will end up with a positive charge.
- If the initially charged object is negatively charged, it will transfer some of its negative charge to the neutral object. Consequently, both objects will end up with a negative charge.
The transfer of charge occurs due to the electrostatic force. Like charges repel each other, and unlike charges attract. In a conductor, charges are free to move. So, when a charged object touches a neutral one, the like charges repel each other, causing a movement of charges until equilibrium is reached.
Examples: Touching a negatively charged rod to a neutral metal sphere will cause electrons to move from the rod to the sphere, leaving both objects negatively charged. A positively charged balloon touched with a neutral metal can cause electrons to move from the can to the balloon, leaving both objects positively charged.
Factors Affecting Charging by Conduction:
- Material: Conductive materials allow for easy transfer of charge.
- Amount of Charge: The initial amount of charge on the charged object determines how much charge will be transferred.
- Duration of Contact: The longer the contact, the more time there is for charges to redistribute.
- Surface Area: A larger contact area can facilitate more charge transfer.
Charging by conduction is a fundamental concept in electrostatics. It demonstrates how charges redistribute themselves to reach a state of equilibrium. This method is widely used in various applications, including grounding electrical systems to safely transfer unwanted charges to the Earth.
In summary, we can say that charging by conduction is a method where a charged object transfers some of its charge to another object through direct contact, resulting in both objects having the same type of charge. This concept is essential for students as it helps explain how charges interact and distribute themselves in conductive materials.
Induction:
Charging an object without direct contact by using a charged object to induce a separation of charges within the neutral object. Charging by induction is a method of charging an object without making direct contact with another charged object. It involves using a charged object to create an electric field that affects a nearby neutral object.
- Bringing a Charged Object Close: A charged object is brought near but not touching a neutral conductor. The presence of the charged object creates an electric field.
- Separation of Charges: The electric field induces a separation of charges within the neutral conductor. Electrons in the conductor will either move towards or away from the charged object, depending on whether it is positively or negatively charged.
- Grounding: Often, the neutral conductor is temporarily connected to the ground, allowing electrons to move between the ground and the conductor. This helps in establishing a charge on the conductor.
- Removing the Connection: The connection to the ground is removed while the charged object is still nearby.
- Retraction of the Charged Object: Finally, the charged object is taken away. The neutral conductor now has a net charge opposite to that of the initially charged object.
If the initial charged object is positively charged, electrons from the ground will move to the neutral conductor, leaving it with a negative charge.
If the initial charged object is negatively charged, electrons will move from the conductor to the ground, leaving the conductor with a positive charge.
The induction process is based on the fundamental principle that opposite charges attract and like charges repel. The electric field from the charged object forces the free electrons in the neutral conductor to move, creating an induced charge.
Examples: Bringing a negatively charged rod near a neutral metal sphere that is grounded can induce a positive charge on the sphere. A positively charged balloon brought near a neutral metal can, which is grounded, will induce a negative charge on the can.
Factors Affecting Charging by Induction:
- Proximity: The closer the charged object is to the neutral conductor, the stronger the induced charge.
- Magnitude of Charge: The greater the charge on the inducing object, the greater the induced charge.
- Duration of Induction: The longer the charged object is held near the neutral conductor, the more pronounced the induction effect.
Polarization:
Polarization is a process that occurs in insulating materials (dielectrics) when they are placed in an electric field. While the charges in these materials cannot move freely, they can shift slightly from their average equilibrium positions.
- Applying an Electric Field: When an external electric field is applied to an insulating material, the negative charges (electrons) are attracted toward the positive side of the field, and the positive charges (nuclei) are repelled toward the negative side.
- Induced Dipoles: This movement creates tiny electric dipoles within the material, with each dipole consisting of a positive charge and a negative charge separated by a small distance.
- Alignment of Dipoles: The dipoles align themselves with the electric field, resulting in a net polarization of the material.
The side of the material facing the positive source of the electric field becomes negatively charged due to the accumulation of electrons.
Conversely, the side facing the negative source becomes positively charged due to the displacement of electrons.
Polarization occurs because the electric field distorts the electron cloud of the atoms or molecules in the material, creating dipoles. These dipoles align with the field, causing a separation of charges within the material.
Examples: When a plastic comb is run through dry hair and brought near small pieces of paper, the paper is attracted to the comb. The comb induces polarization in the paper pieces, and the opposite charges attract.
A balloon rubbed against clothing and then placed near a wall will stick to the wall. The wall becomes polarized, with the side closest to the balloon becoming oppositely charged, leading to attraction.
Factors Affecting Polarization:
- Strength of the Electric Field: A stronger field will induce a greater degree of polarization.
- Properties of the Material: Different materials have varying abilities to become polarized, known as their polarizability.
- Shape of the Material: The shape can influence how the charges are distributed during polarization.
Polarization is a key concept in understanding the behavior of dielectric materials in electric fields. It has practical applications in capacitors, where dielectrics are used to increase capacitance, and in various electronic devices.
In summary, electric charge by polarization is a method where an insulating material becomes polarized in the presence of an external electric field, leading to a separation of charges within the material.
Uses of Electric Charge
Electric charge, a fundamental property of matter, has numerous applications that are pivotal in various fields. Here are some uses of electric charges;
Electronics: Electric charges are the basis of all electronic devices, from the simplest circuits to complex computer systems. They allow for the control and manipulation of electrical signals.
Electrostatics: The study and application of static electricity, where charges are used in devices like photocopiers and paint sprayers.
Medical Devices: Charges play a crucial role in medical diagnostics and treatment, such as in electrocardiograms (ECGs) and defibrillators.
Energy Storage: In batteries and capacitors, electric charges are stored and used as a source of energy.
Sensors: Touch screens and piezoelectric sensors rely on the interaction of electric charges to function.
Scientific Research: Particle accelerators use electric charges to study the fundamental particles of matter.
Industrial Processes: Processes like electroplating and electrolysis involve the transfer of electric charges.
Daily Life: Static electricity is a common phenomenon experienced in daily life, from static clinging to clothes to lightning during storms.
Solved Examples
Example 1: Two point charges (q1 = 3 µC) and (q2 = -5 µC) are placed 2 meters apart in the air. Calculate the magnitude and direction of the electric force between them.
Solution: Given; (q1 = 3 µC ), (q2 = -5 µC ), (r = 2m), (k = 9 × 109, N ⋅ m2/C2 )
Magnitude of electric force:
\(\displaystyle F = \frac{k |q_1 q_2|}{r^2} \)
\(\displaystyle F = \frac{9 \times 10^9 \times |3 \times 10^{-6} \times -5 \times 10^{-6}|}{(2)^2} \)
\(\displaystyle F = \frac{9 \times 10^9 \times 15 \times 10^{-12}}{4} \)
\(\displaystyle F = \frac{135 \times 10^{-3}}{4}\)
\(\displaystyle F = 33.75 \times 10^{-3}\)
\(\displaystyle F = 3.375 \times 10^{-2} \, N \)
The direction of the force is attractive since the charges are of opposite sign.
Example 2: Calculate the electric field intensity at a distance of 4 meters from a point charge of (2 µC ).
Solution: Given; (q = 2 µC ), (r = 4m), (k = 9 ×109 N ⋅ m2/C2 )
Electric field intensity:
\(\displaystyle E = \frac{k|q|}{r^2} \)
\(\displaystyle E = \frac{9 \times 10^9 \times |2 \times 10^{-6}|}{(4)^2} \)
\(\displaystyle E = \frac{18 \times 10^{-3}}{16} \)
\(\displaystyle E = 1.125 \times 10^{-3} \, N/C \)
Therefore, the electric field intensity at a distance of 4 meters from the point charge is (1.125 × 10-3 N/C ).
Example 3: Calculate the electric potential energy of a system consisting of two charges ( q1 = 6 µC) and (q2 = -4 µC ) placed 3 meters apart in the air.
Solution: Given; (q1 = 6 µC ), (q2 = -4 µC ), (r = 3m), \(\displaystyle k = 9 \times 10^9 \, N \cdot m^2/C^2 \)
Electric potential energy:
\(\displaystyle U = \frac{k |q_1 q_2|}{r} \)
\(\displaystyle U = \frac{9 \times 10^9 \times |6 \times 10^{-6} \times -4 \times 10^{-6}|}{3} \)
\(\displaystyle U = \frac{9 \times 10^9 \times 24 \times 10^{-12}}{3} \)
\(\displaystyle U = \frac{216 \times 10^{-3}}{3} \)
\(\displaystyle U = 72 \times 10^{-3} \)
\(\displaystyle U = 7.2 \times 10^{-2} \, J \)
Therefore, the electric potential energy of the system is \(\displaystyle 7.2 \times 10^{-2} \, J \).
Example 4: Calculate the electric field intensity at a point 10 cm away from the center of a uniformly charged rod of length 20 cm and total charge (5 µC).
Solution: Given; (Q = 5 µC ), (L = 20cm = 0.2m), (r = 10cm = 0.1m), \(\displaystyle k = 9 \times 10^9 \, N \cdot m^2/C^2 \)
Electric field intensity due to a uniformly charged rod:
\(\displaystyle E = \frac{kQ}{2L} \left( \frac{1}{\sqrt{r^2 + \left(\frac{L}{2}\right)^2}} – \frac{1}{\sqrt{r^2 + \left(\frac{L}{2}\right)^2}} \right) \)
\(\displaystyle E = \frac{9 \times 10^9 \times 5 \times 10^{-6}}{2 \times 0.2} \left( \frac{1}{\sqrt{(0.1)^2 + (0.1)^2}} – \frac{1}{\sqrt{(0.1)^2 + (0.1)^2}} \right) \)
\(\displaystyle E = \frac{45 \times 10^{-3}}{0.4} \left( \frac{1}{\sqrt{0.02}} – \frac{1}{\sqrt{0.02}} \right) \)
\(\displaystyle E = \frac{112.5}{0.4} \times 0 \)
E = 0
Therefore, the electric field intensity at a point 10 cm away from the center of the rod is (0 N/C ) due to symmetry.
Example 5: Calculate the electric potential at a point 5 cm away from the surface of a charged sphere with a radius of 10 cm and total charge (8 µC ).
Solution: Given: (Q = 8 µC ), (r = 10cm = 0.1m), (d = 5cm = 0.05m), \(\displaystyle k = 9 \times 10^9 \, N \cdot m^2/C^2 \)
Electric potential due to a uniformly charged sphere:
\(\displaystyle V = \frac{kQ}{r} \)
\(\displaystyle V = \frac{9 \times 10^9 \times 8 \times 10^{-6}}{0.1} \)
\(\displaystyle V = \frac{72 \times 10^{-3}}{0.1} \)
\(\displaystyle V = 720 \times 10^{-3} \)
\(\displaystyle V = 72 \, V \)
Therefore, the electric potential at a point 5 cm away from the surface of the sphere is (72 V).
Example 6: Calculate the electric field intensity at a point on the axis of a uniformly charged ring of radius 0.1 m and total charge (10 µC ), located 0.2 m away from the center of the ring.
Solution: Given; (Q = 10 µC ), (r = 0.1m ), (d = 0.2m ), \(\displaystyle k = 9 \times 10^9 \, N \cdot m^2/C^2 \)
Electric field intensity due to a uniformly charged ring:
\(\displaystyle E = \frac{kQd}{(d^2 + r^2)^{3/2}} \)
\(\displaystyle E = \frac{9 \times 10^9 \times 10 \times 10^{-6} \times 0.2}{(0.2^2 + 0.1^2)^{3/2}} \)
\(\displaystyle E = \frac{180 \times 10^{-6}}{(0.04 + 0.01)^{3/2}} \)
\(\displaystyle E = \frac{180 \times 10^{-6}}{(0.05)^{3/2}} \)
\(\displaystyle E = \frac{180 \times 10^{-6}}{0.0354} \)
\(\displaystyle E ≈ 5084.75 \times 10^{-6} \)
\(\displaystyle E ≈ 5.08475 \times 10^{-2} \, N/C \)
Therefore, the electric field intensity at a point on the axis of the charged ring is approximately \(\displaystyle 5.08475 \times 10^{-2} \, N/C \).
FAQs
What is electric charge, and how is it defined in physics?
Electric charge is a fundamental property of matter that determines how it interacts with electric and magnetic fields. It comes in two types: positive and negative. In physics, electric charge is defined as the physical property of matter that causes it to experience a force when placed in an electromagnetic field
Can you explain the difference between positive and negative electric charges?
Positive electric charge is associated with protons, while negative electric charge is associated with electrons. Protons have a positive charge, and electrons have a negative charge. Objects with an excess of protons have a net positive charge, while those with an excess of electrons have a net negative charge.
How is electric charge quantified, and what are its units?
Electric charge is quantified in coulombs (C), named after French physicist Charles-Augustin de Coulomb. One coulomb is equal to the charge transported by a constant current of one ampere in one second. The elementary charge, (e), is the charge of a single proton or electron and is approximately (1.602 × 10-19) coulombs.
What are some common materials or objects that can become charged?
Various materials can become charged through friction, contact, or induction. Common examples include plastics, rubber, glass, and metals. When two different materials are rubbed together, electrons may be transferred between them, resulting in one material becoming positively charged and the other negatively charged.
How do like and unlike charges interact with each other?
Like charges repel each other, meaning two positively charged objects or two negatively charged objects will push each other away. Unlike charges attract each other, so a positively charged object will be attracted to a negatively charged object.
Can electric charge be created or destroyed, and how does it behave in isolated systems?
According to the law of conservation of charge, electric charge cannot be created or destroyed, only transferred from one object to another. In isolated systems, the total electric charge remains constant over time, even if charges redistribute themselves within the system.
What are the practical applications of understanding electric charge in modern technology?
Understanding electric charge is crucial in various fields, including electronics, telecommunications, and power generation. It forms the basis of technologies such as batteries, capacitors, electric circuits, and electromagnetism, enabling the development of devices ranging from smartphones and computers to power grids and electric vehicles.