The journey to understanding the electromagnetic spectrum began with early studies of light. In the 17th century, Isaac Newton made a significant discovery when he passed white light through a glass prism. He observed that the light split into a spectrum of colors: red, orange, yellow, green, blue, indigo, and violet. This demonstrated that white light is composed of different colors, which we now know are different wavelengths of light.
In the early 19th century, Thomas Young and Augustin-Jean Fresnel conducted experiments that showed light behaves as a wave. Young’s double-slit experiment demonstrated that light waves can interfere with each other, creating patterns of light and dark bands. Fresnel’s work further solidified the wave theory of light.
A breakthrough came in the mid-19th century with James Clerk Maxwell. He developed a set of equations, now known as Maxwell’s equations, which described how electric and magnetic fields interact and propagate as waves through space. Maxwell’s equations predicted that these waves travel at the speed of light and that light itself is an electromagnetic wave.
In the late 19th century, Heinrich Hertz conducted experiments that confirmed Maxwell’s predictions. Hertz generated electromagnetic waves in the laboratory and detected them at a distance, proving that such waves could travel through space. These waves were later named radio waves.
Expanding the Spectrum: Following Hertz’s discovery, scientists began to explore other forms of electromagnetic radiation:
- Wilhelm Röntgen discovered X-rays in 1895 while experimenting with cathode rays. He noticed that a new type of ray could pass through solid objects and create images of bones.
- Henri Becquerel discovered natural radioactivity in 1896, which led to the discovery of gamma rays by Paul Villard in 1900.
- Ultraviolet light was identified through experiments with sunlight, revealing that there were invisible rays beyond the violet end of the visible spectrum.
- Infrared radiation was discovered by William Herschel in 1800 when he used a thermometer to measure the heat beyond the red end of the visible spectrum.
By the early 20th century, scientists understood that the electromagnetic spectrum included a wide range of wavelengths, from very short gamma rays to very long radio waves. This expanded view of the spectrum allowed for the development of new technologies and a deeper understanding of the universe.
Today, we know that the electromagnetic spectrum encompasses all types of electromagnetic radiation, each with its unique properties and uses. This understanding has led to advancements in communication, medicine, astronomy, and many other fields, fundamentally transforming how we live and interact with the world.
What is an Electromagnetic Spectrum?
When we talk about the electromagnetic spectrum, we’re referring to this entire collection of waves, from the longest radio waves that can be as long as mountains to the shortest gamma rays that are smaller than atoms, and everything in between. It encompasses all frequencies and wavelengths of electromagnetic radiation.
The electromagnetic spectrum is like a vast ocean of different kinds of light waves, some visible to our eyes and others invisible. Imagine you’re at a beach, and you can see waves of different sizes coming towards the shore. Similarly, the electromagnetic spectrum consists of waves that are all around us, but instead of water, these are waves of energy moving through space.
These waves are called electromagnetic waves because they have both electric and magnetic parts to them. They travel at the speed of light, which is about 299,792 kilometers per second! Now, just like ocean waves can have long or short distances between each wave peak, electromagnetic waves also have different lengths between their peaks, which we call wavelengths.
The spectrum includes all types of electromagnetic radiation, ranging from those with really long wavelengths (like radio waves) to those with super short wavelengths (like gamma rays). The amazing thing is that this spectrum covers a wide range of energies and wavelengths, but our eyes can only detect a tiny part of it, which we call visible light. It’s a continuous range of energy that’s fundamental to many technologies and natural phenomena in our world.
Electromagnetic Waves and its Representation
Electromagnetic waves are oscillations of electric and magnetic fields that travel through space. Imagine you’re at a concert, and you can feel the music vibrating through the air. These vibrations travel as waves from the stage to your ears. Similarly, electromagnetic waves are like invisible vibrations that travel through space and carry energy with them.
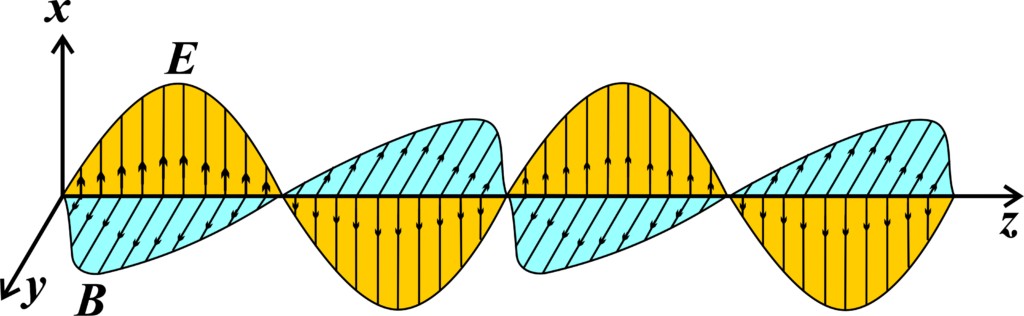
Now, how do we represent these invisible waves? We use two main characteristics: wavelength and frequency.
- Wavelength is the distance between two consecutive peaks or troughs of a wave. It’s like measuring the distance between two high points of a wave in the ocean. In diagrams, the wavelength is represented by the Greek letter lambda (λ).
- Frequency is the number of waves that pass a certain point in one second. If you were counting the number of times the water rises and falls at the beach in one second, that’s like measuring the frequency of ocean waves. In equations, frequency is represented by the letter ‘f’.
These two characteristics are related by the speed of light (c), which is a constant. The relationship is given by the equation:
\(\displaystyle c = \lambda \times f \)
This means that if you know the wavelength of an electromagnetic wave, you can figure out its frequency, and vice versa, as long as you know the speed of light.
So, when physicists talk about electromagnetic waves, they often describe them by their wavelength or frequency. This helps us understand and categorize the different types of waves in the electromagnetic spectrum, from radio waves with long wavelengths to gamma rays with very short wavelengths.
And that’s the essence of electromagnetic waves and how we represent them. It’s all about the invisible energy traveling through space, and the way we measure and understand that energy is through its wavelength and frequency.
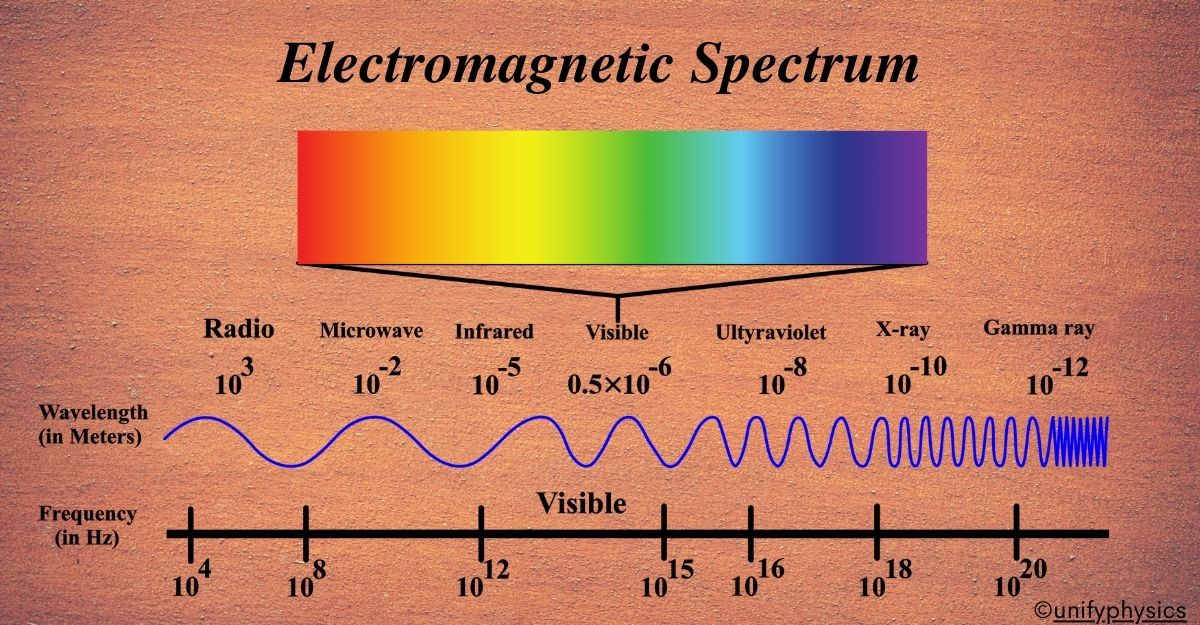
Electromagnetic Spectrum
The spectrum is typically divided into regions based on wavelength or frequency. From longest wavelength to shortest, it includes radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, and gamma rays.
Think of the electromagnetic spectrum as a grand piano. Each key on the piano represents a different type of electromagnetic wave, with the keys on the left being the low-frequency waves and the keys on the right being the high-frequency waves. Just like a piano can play a wide range of notes, the electromagnetic spectrum includes a wide range of waves.
Now, these waves aren’t sound waves; they’re all forms of light! Yes, even the ones we can’t see with our eyes. The electromagnetic spectrum is the entire collection of these light waves, organized by their frequency and wavelength.
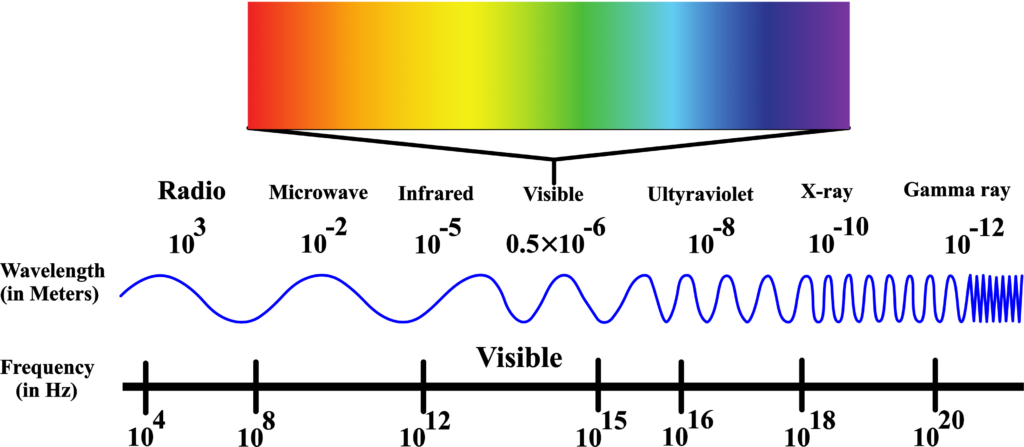
We have the radio waves from the left side of our piano. These are like the deep, low notes. They have the longest wavelengths and the lowest frequencies. These have the longest wavelengths, from about a millimeter to hundreds of meters or more, and the lowest frequencies, from less than 3 kHz to about 300 GHz. Radio waves are used for broadcasting music and talk shows on your radio.
Next up, we have microwaves. They’re a bit higher in pitch on our piano. Wavelengths range from one millimeter to one meter, with frequencies between 300 MHz (0.3 GHz) and 300 GHz. Microwaves have shorter wavelengths than radio waves and are used in your microwave oven to heat food.
Moving further to the right, we find infrared waves. These are like the warm notes in the middle of the piano. Wavelengths range from 700 nm (nanometers) to 1 mm, and frequencies range from about 300 GHz to 400 THz (terahertz). We can feel infrared waves as heat, which are used in things like remote controls.
The middle of the piano represents visible light. This is the part of the spectrum we can actually see, and it’s made up of all the colors of the rainbow. Each color is a different note, from red to violet. This small part of the spectrum includes wavelengths from about 400 nm (violet) to 700 nm (red), with frequencies ranging from 430 THz to 750 THz.
As we move to the higher notes, we get to ultraviolet light. Wavelengths span from 10 nm to 400 nm, and frequencies range from 30 PHz (petahertz) to 750 THz. These waves have more energy and can give you a sunburn if you’re not careful.
Even higher are the X-rays. Just like the high notes on a piano can be sharp, X-rays have a lot of energy and can pass through your body, which is why doctors use them to look at your bones. These have wavelengths ranging from 0.01 nm to 10 nm, with corresponding frequencies from 30 PHz to 30 EHz (exahertz).
Finally, the highest notes on our piano are the gamma rays. These have the shortest wavelengths and the highest frequencies. The shortest wavelengths, less than 0.01 nm, and the highest frequencies, over 30 EHz. They carry a lot of energy and are used in medicine to treat cancer.
So, the electromagnetic spectrum is like a keyboard of light, with each type of wave having its place and purpose, just like each note on a piano creates a different sound. And that’s the electromagnetic spectrum – a beautiful symphony of light waves that are all around us, each playing its unique part in the universe!
Electromagnetic Waves in the Electromagnetic Spectrum
The electromagnetic spectrum is a continuous range of electromagnetic waves, sorted by their frequency and wavelength. Here’s a breakdown of the different types of waves:
Radio Waves: These are the gentle giants of the spectrum, with the longest wavelengths and lowest frequencies. They’re used for broadcasting radio and TV signals, and even by stars and gases in space to send out their own ‘messages’.
Microwaves: A bit more energetic, microwaves have shorter wavelengths than radio waves. They’re used in your microwave oven to heat food and by astronomers to study the structure of galaxies and stars.
Infrared Rays: Often called heat waves, infrared rays are emitted by hot objects. They’re used in night vision goggles to see in the dark and by remote controls to change the channel on your TV.
Visible Rays: These are the waves you can see, spanning all the colors of the rainbow. They help us perceive the world around us and are emitted by various light sources like bulbs and LEDs.
Ultraviolet Rays: With even shorter wavelengths, ultraviolet rays can tan your skin and are used in water purifiers to kill microbes. They’re also used in medical procedures like LASIK eye surgery.
X-rays: These high-energy waves can pass through soft tissue and are used in medicine to look at bones or teeth. They’re also used at airport security to check your luggage.
Gamma Rays: The most energetic waves in the spectrum, gamma rays are used in medicine to treat cancer and by astronomers to explore the universe’s most explosive events.
Also Read: Electromagnetic Waves
Formulas for the Electromagnetic Radiation
Speed of Electromagnetic Waves (c): The speed of light in a vacuum is a fundamental constant and is given by:
\(\displaystyle c = 3 \times 10^8 \text{ m/s} \)
Relationship Between Wavelength (λ) and Frequency (f): The wavelength and frequency of an electromagnetic wave are inversely proportional to each other and related by the speed of light:
\(\displaystyle c = \lambda \times f \)
Energy of a Photon (E): The energy of a photon is directly proportional to its frequency and is given by Planck’s equation:
\(\displaystyle E = h \times f \)
where (h) is Planck’s constant (\(\displaystyle 6.626 \times 10^{-34} \text{ J s} \)).
Momentum of a Photon (p): A photon also carries momentum, which is given by:
\(\displaystyle p = \frac{E}{c} = \frac{h}{\lambda} \)
Electric Field (E) and Magnetic Field (B) Amplitude: In an electromagnetic wave, the electric field and magnetic field amplitudes are related by the speed of light:
\(\displaystyle E = c \times B \)
The intensity of Electromagnetic Radiation (I): The intensity is the power per unit area carried by a wave and is related to the amplitude of the electric field:
\(\displaystyle I = \frac{1}{2} \epsilon_0 c E^2 \)
where (\(\displaystyle \epsilon_0 \)) is the permittivity of free space (\(\displaystyle 8.854 \times 10^{-12} \text{ F/m} \)).
These formulas are fundamental to understanding how electromagnetic waves behave and interact with matter.
Significance of the Electromagnetic Spectrum
Understanding the electromagnetic spectrum is crucial for many technologies, such as communication, medical imaging, and astronomy. It also helps us understand the physical properties of light and other forms of radiation. Here’s why it’s so important:
- Communication: Without the electromagnetic spectrum, we wouldn’t have Wi-Fi, smartphones, or satellite TV. Radio waves, a part of the spectrum, keep us connected with the world.
- Healthcare: X-rays and gamma rays help doctors look inside our bodies without surgery, and they can even treat cancer. That’s the power of the electromagnetic spectrum at work in medicine.
- Safety: Ultraviolet light can kill germs, and X-rays can check our luggage at airports for dangerous items. The spectrum helps keep us safe in ways we often don’t see.
- Science and Discovery: Astronomers use the entire spectrum to study the universe. From radio waves emitted by distant galaxies to gamma rays from black holes, the spectrum is a tool for unlocking the mysteries of space.
- Everyday Life: Microwaves cook our food, infrared technology controls our TVs, and visible light lets us see the world in color. The spectrum is part of almost everything we do.
Electromagnetic Spectrum Wavelength and Frequency Table
This table provides a concise overview of the different types of electromagnetic radiation, their place in the spectrum, and how they interact with various materials.
| Type of Radiation | Frequency Range (Hz) | Wavelength Range | Interaction with Matter |
|---|---|---|---|
| Gamma-rays | 1020 – 1024 | < 10-12 m | Can damage living cells, used in cancer treatment, pass through most materials |
| X-rays | 1017 – 1020 | 1 nm – 1 pm | Absorbed by bone, pass through soft tissue, used in medical imaging |
| Ultraviolet | 1015 – 1017 | 400 nm – 1 nm | Causes sunburn, can damage eyes, used for sterilization |
| Visible | 4 x 1014 – 7.5 x 1014 | 750 nm – 400 nm | Reflected and absorbed by objects, allows us to see colors |
| Near-infrared | 1 x 1014 – 4 x 1014 | 2.5 μm – 750 nm | Emitted as heat, used in remote controls and thermal imaging |
| Infrared | 1013 – 1014 | 25 μm – 2.5 μm | Felt as heat, used in heaters and night vision equipment |
| Microwaves | 3 x 1011 – 1013 | 1 mm – 25 μm | Absorbed by water molecules, used in microwave ovens and satellite communication |
| Radio waves | < 3 x 1011 | > 1 mm | Used for broadcasting and communication, can travel long distances |
Practical Applications of Electromagnetic Waves
Each type of electromagnetic wave has its unique applications that impact our daily lives, from the way we communicate to the way we receive medical care.
- Radio Waves: These are used for communication over long distances, such as broadcasting radio and television programs. They’re like the invisible messengers carrying your favorite songs and shows through the air.
- Microwaves: Not just for heating your food, microwaves are also crucial in satellite communications, linking people across the globe almost instantly.
- Infrared: Beyond keeping us warm, infrared waves are used in electrical heaters, cooking, and even in infrared cameras that can see heat, which is pretty cool (or hot!).
- Visible Light: The light we see every day is also used in fiber optic communications, sending data at the speed of light under the oceans and across continents.
- Ultraviolet: These waves have medical applications and are also used in energy-efficient lamps and for getting that summer tan (with sunscreen, of course!).
- X-rays: X-rays let doctors peer inside your body without surgery, helping to diagnose and treat various medical conditions.
- Gamma Rays: In medicine, gamma rays are powerful tools for treating cancer, and targeting and destroying cancer cells.
Sample Questions
Question: Arrange the following types of electromagnetic waves in increasing order of their wavelengths: X-rays, microwaves, visible light, infrared radiation, and radio waves.
Solution: The electromagnetic spectrum orders waves by their wavelengths, from shortest to longest. Here’s the arrangement:
- X-rays: Shortest wavelength
- Visible light: Depends on color; typically shorter than microwaves but longer than X-rays.
- Infrared radiation: Longer wavelength than visible light
- Microwaves: Longer than infrared radiation
- Radio waves: Longest wavelength
Question: Calculate the frequency of electromagnetic radiation with a wavelength of (4 × 10-7) meters.
Solution: The frequency (f) of electromagnetic waves can be calculated using the formula:
\(\displaystyle f = \frac{c}{\lambda}\)
\(\displaystyle f = \frac{3 \times 10^8}{4 \times 10^{-7}} \)
\(\displaystyle f = 7.5 \times 10^{14} \, \text{Hz} \)
Question: Calculate the energy of a photon with a frequency of (6 × 1014) Hz.
Solution: The energy (E) of a photon can be calculated using the formula:
E = hf
\(\displaystyle E = 6.626 \times 10^{-34} \times 6 \times 10^{14} \)
\(\displaystyle E = 3.9756 \times 10^{-19} \, \text{J} \)
Question: Explain how ultraviolet radiation from the sun can damage the skin.
Solution: Ultraviolet (UV) radiation from the sun has a shorter wavelength and higher energy compared to visible light. When UV radiation reaches the skin, it can penetrate the outer layers and damage the DNA in skin cells. This damage can lead to mutations and increase the risk of skin cancer. Additionally, UV radiation can cause premature aging of the skin, including wrinkles and age spots.
FAQs
What determines the position of electromagnetic waves on the spectrum?
The position of electromagnetic waves on the spectrum is determined by their frequency or wavelength. Waves with lower frequencies, such as radio waves, are positioned at the left end of the spectrum, while waves with higher frequencies, like gamma rays, are positioned at the right end.
How do the wavelengths of electromagnetic waves relate to their energies?
Electromagnetic waves with shorter wavelengths have higher energies, while those with longer wavelengths have lower energies. This relationship is described by the equation \(\displaystyle E = \frac{hc}{\lambda} \).
What physical phenomena determine the boundaries between different regions of the electromagnetic spectrum?
The boundaries between different regions of the electromagnetic spectrum are determined by physical phenomena such as absorption, emission, and scattering. For example, the boundary between infrared and visible light is defined by the range of wavelengths where the human eye is sensitive to electromagnetic radiation.
How do electromagnetic waves interact with matter as they traverse through different mediums?
Electromagnetic waves interact with matter through processes such as reflection, refraction, absorption, and transmission. The extent of interaction depends on the properties of the medium and the wavelength of the waves. For example, visible light waves are often transmitted through glass but absorbed by opaque materials.
What role does the speed of light play in defining the electromagnetic spectrum?
The speed of light in a vacuum (c) is a fundamental constant that determines the propagation speed of all electromagnetic waves. The spectrum is organized based on the frequency or wavelength of waves relative to the speed of light. This speed is approximately ( 3 × 108 ) meters per second.
How do astronomers use the different regions of the electromagnetic spectrum to study celestial objects?
Astronomers utilize various regions of the electromagnetic spectrum, from radio waves to gamma rays, to study different aspects of celestial objects. Each region provides unique information about the temperature, composition, motion, and other properties of astronomical objects.
Can electromagnetic waves with frequencies or wavelengths outside the visible spectrum still be detected or observed?
Yes, electromagnetic waves with frequencies or wavelengths outside the visible spectrum can still be detected or observed using specialized instruments. For example, radio telescopes detect radio waves from distant galaxies, and X-ray detectors capture X-rays emitted by celestial objects.