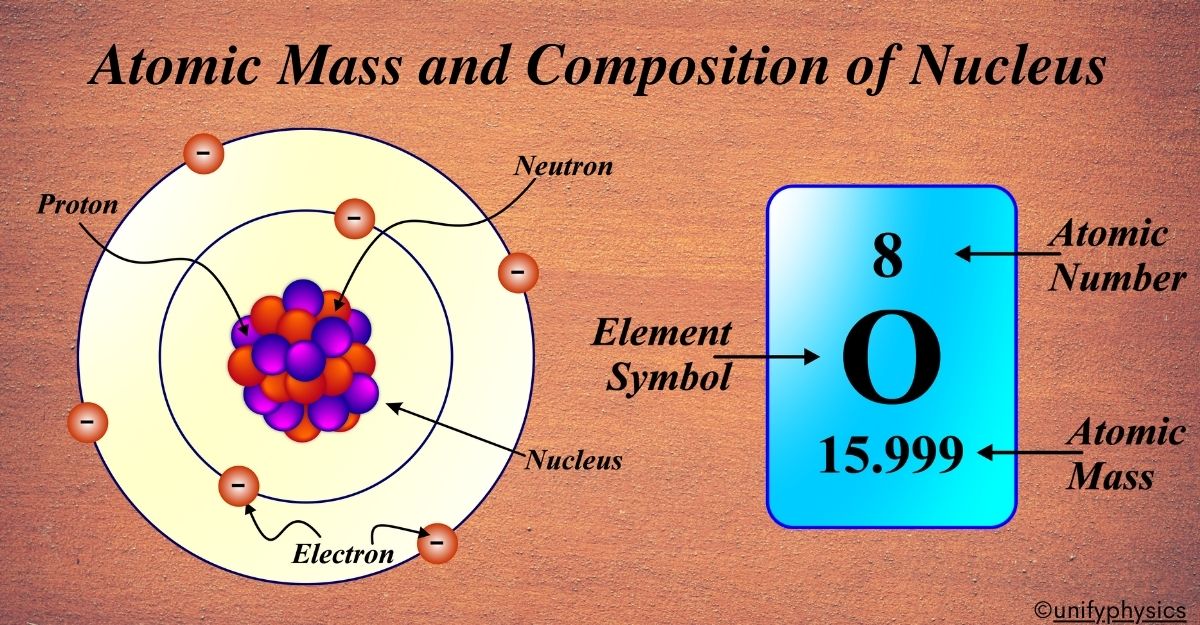
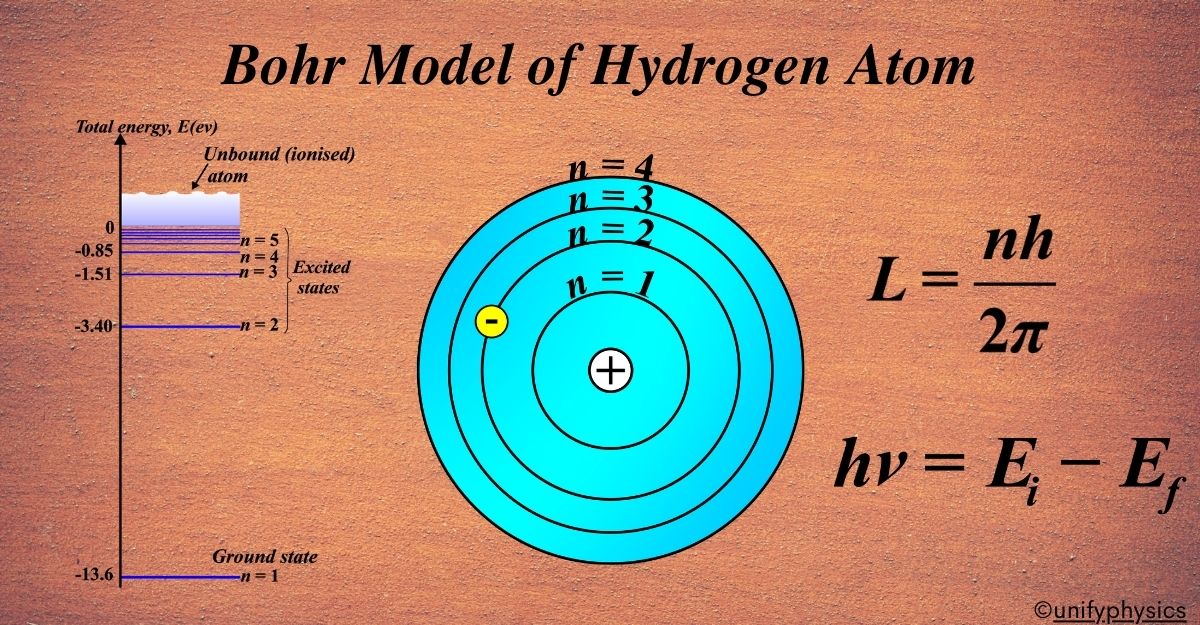
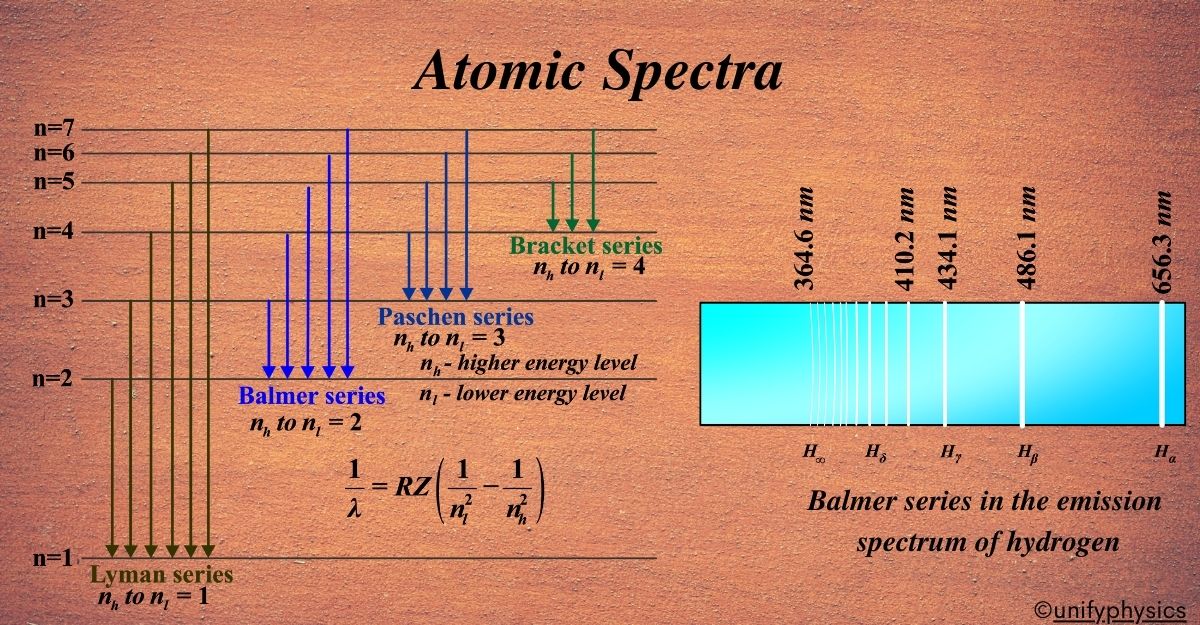
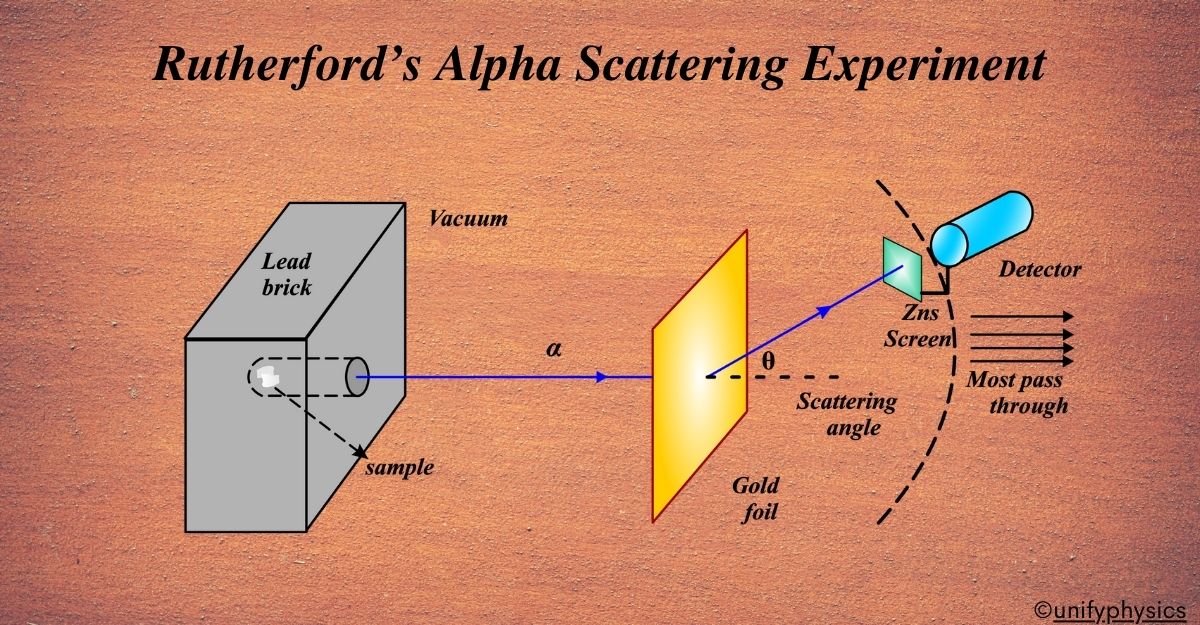
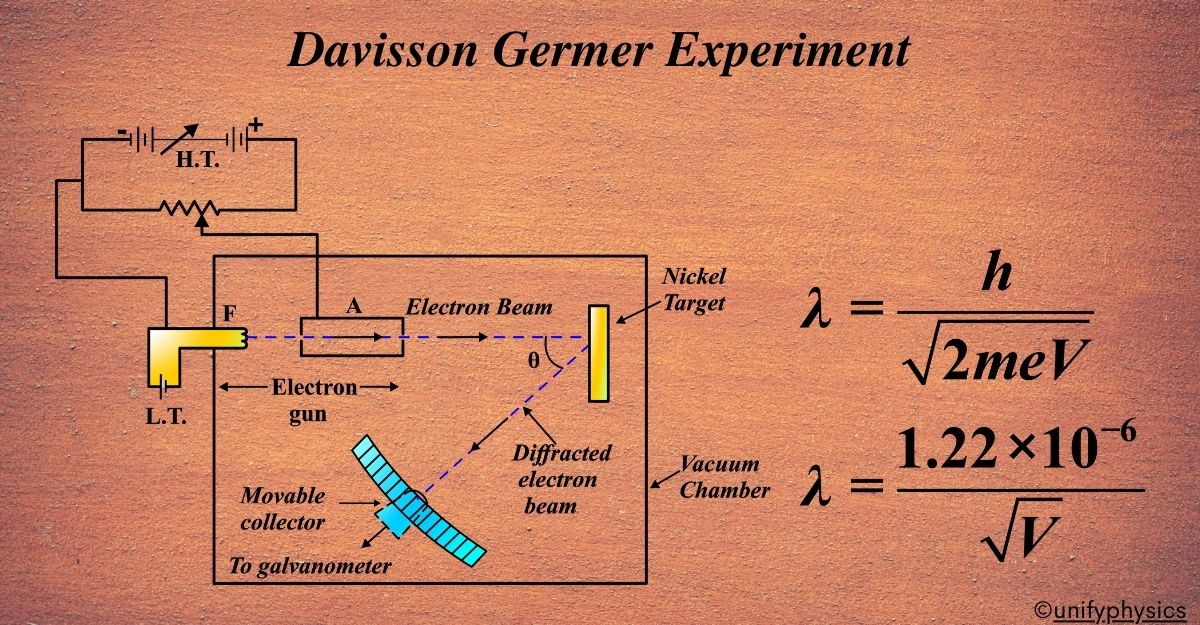
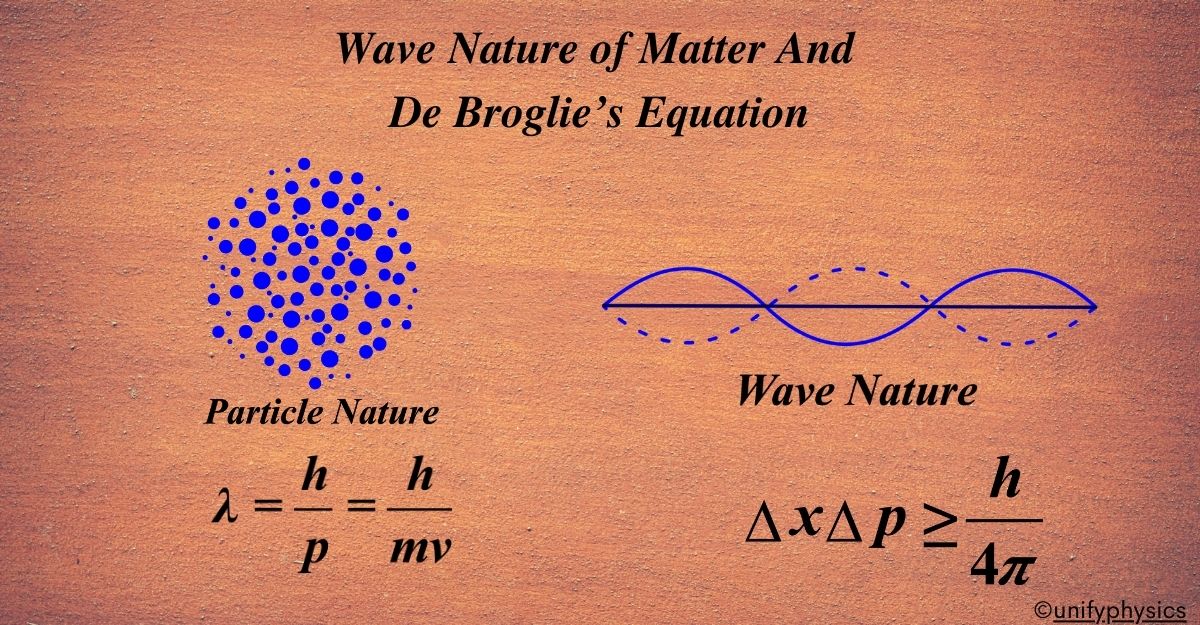
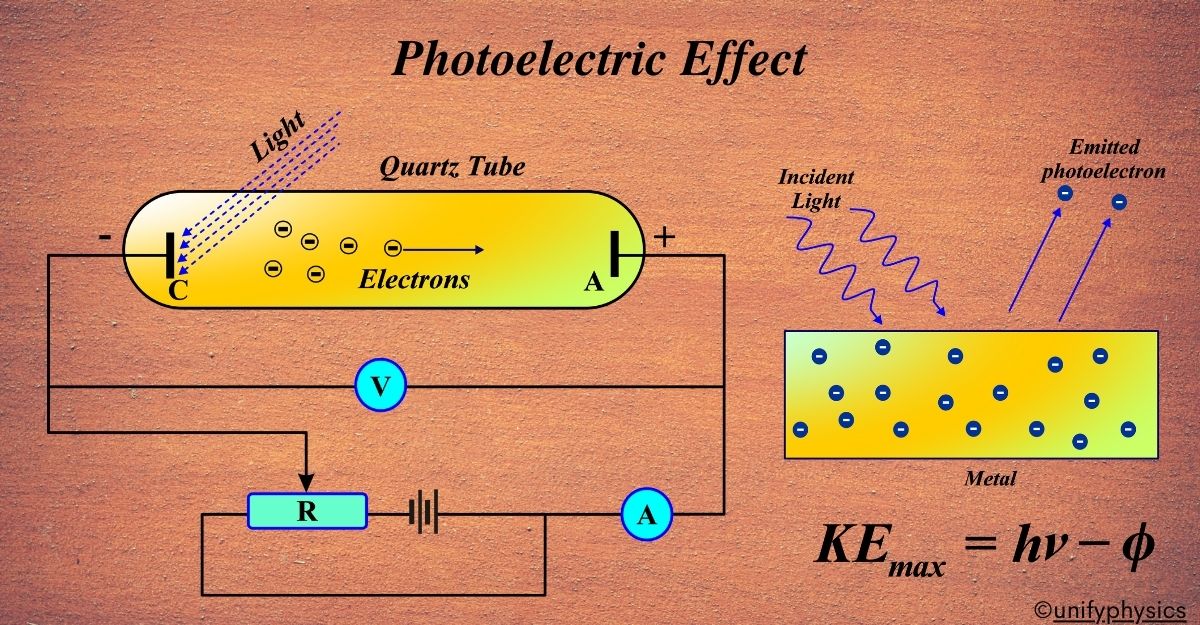
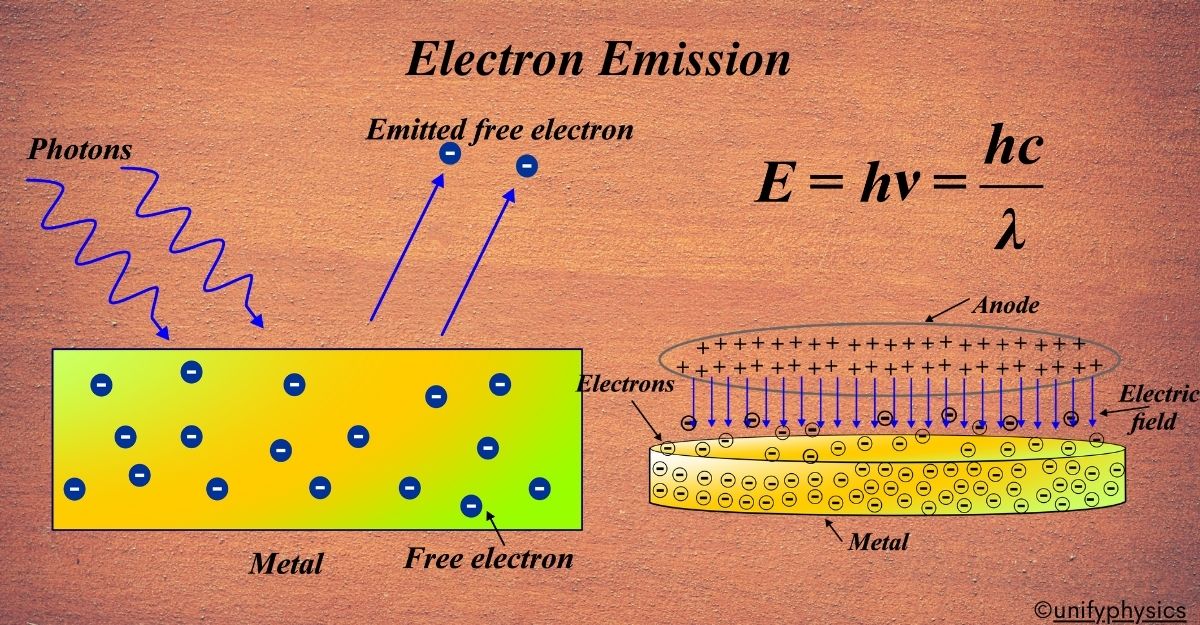
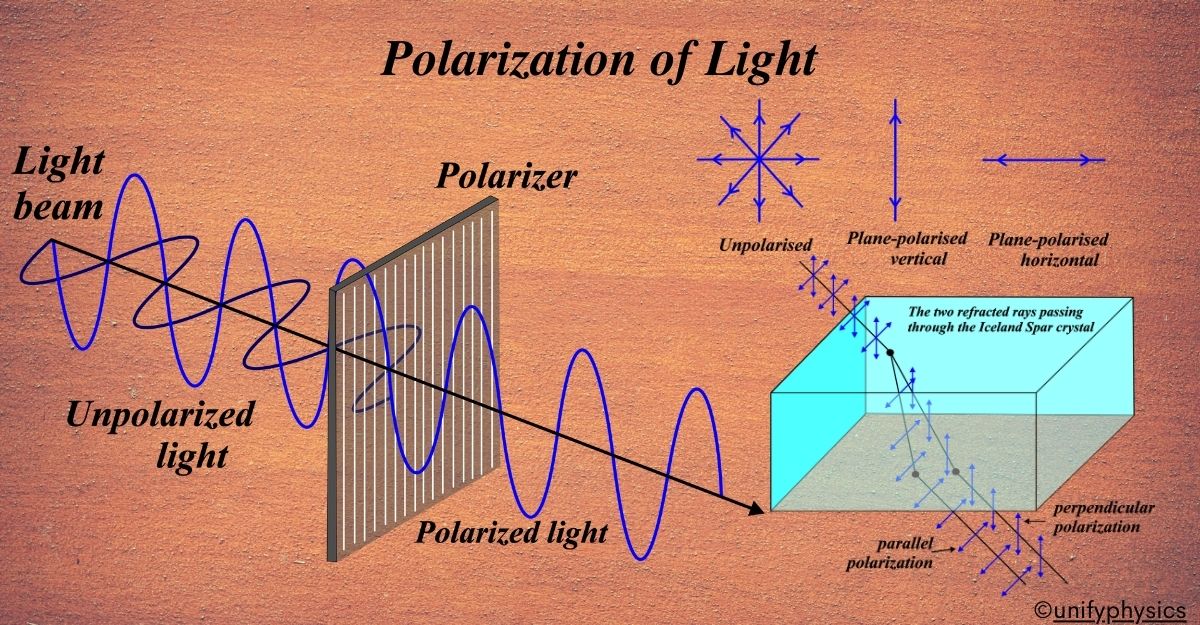
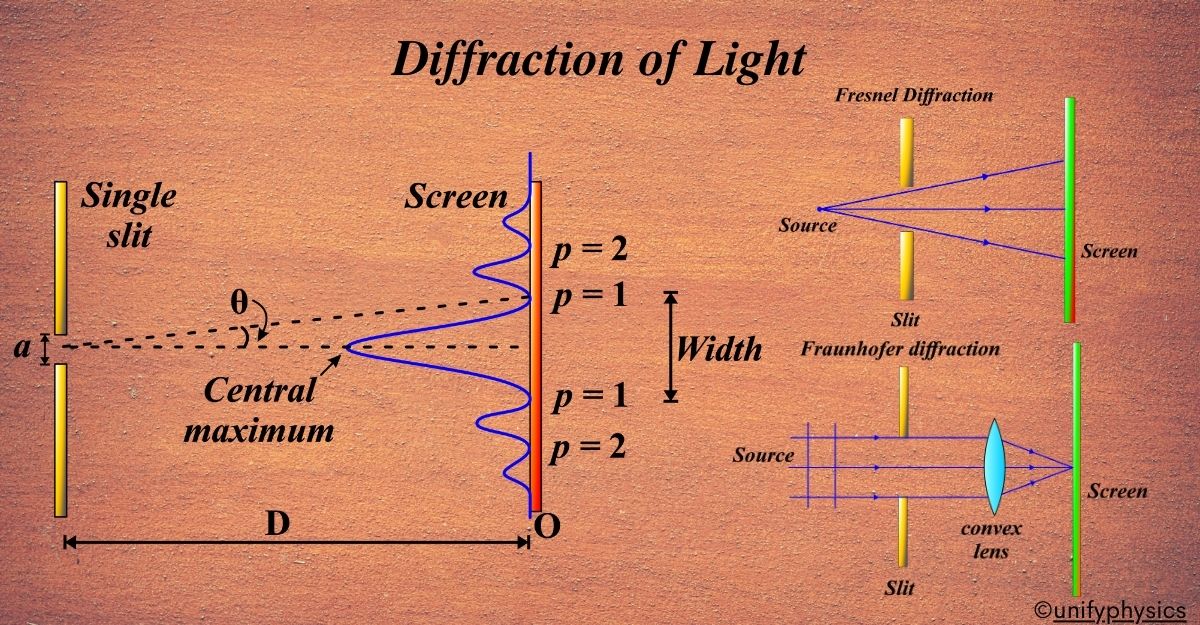
Atomic Mass and Composition of Nucleus
The idea of atoms as the fundamental building blocks of matter dates back to ancient Greece, but it wasn’t until the 19th century that the concept began to take a scientific form. John Dalton, in the early 1800s, proposed that each element is composed of unique atoms and that chemical reactions involve the rearrangement of … Read more