The concept of latent heat is like a hidden chapter in the story of temperature and heat. It’s the secret agent of energy changes, working behind the scenes during phase changes. People have always known that substances like water can exist in different states—solid, liquid, and gas. However, the understanding of how and why these changes occurred took a while to develop.
The story of latent heat heats up with a Scottish scientist named Joseph Black in the mid-18th century. He was the one who first introduced the idea of latent heat between 1750 and 1762. Black was working with Scotch bourbon producers to figure out the best mix of fuel and water for distilling.
Black noticed that when the ice melted or water boiled, the temperature didn’t change even though heat was being added. This led him to propose the concept of latent heat—the heat absorbed or released during a change of state that doesn’t change the temperature.
Black’s work laid the groundwork for the field of thermodynamics. His ideas helped other scientists to understand that heat is a form of energy and that energy conservation is a fundamental principle of nature.
Today, the concept of latent heat is crucial in many areas, from meteorology to the design of heat engines and refrigeration systems. It’s a concept that has gone from a curious observation to a cornerstone of modern science.
What is Latent Heat?
Latent Heat is the energy absorbed or released by a substance during a phase change—like melting, freezing, or boiling—without changing its temperature. The word “latent” itself means hidden or concealed, indicating that this heat energy is not evident in the temperature of the substance but rather hidden within its molecular structure during a phase change. It’s the hidden energy that helps substances change their state while the temperature remains constant.
Latent Heat is like a secret agent of energy. It works undercover during a phase change, which is when a substance changes from one state of matter to another, like ice melting into water or water boiling into steam. This energy doesn’t show up as a temperature change; instead, it’s busy helping molecules break free from their phase. It’s like the energy is ‘latent’ or waiting to be used, hence the name ‘Latent Heat’.
Imagine a group of people (molecules) tightly holding hands in a crowded room (solid). To move freely (liquid), they need extra energy to let go of each other’s hands. Latent Heat is that energy boost that helps them let go without making the room hotter or colder. It’s the energy needed to overcome the attraction between the molecules without changing the overall temperature.
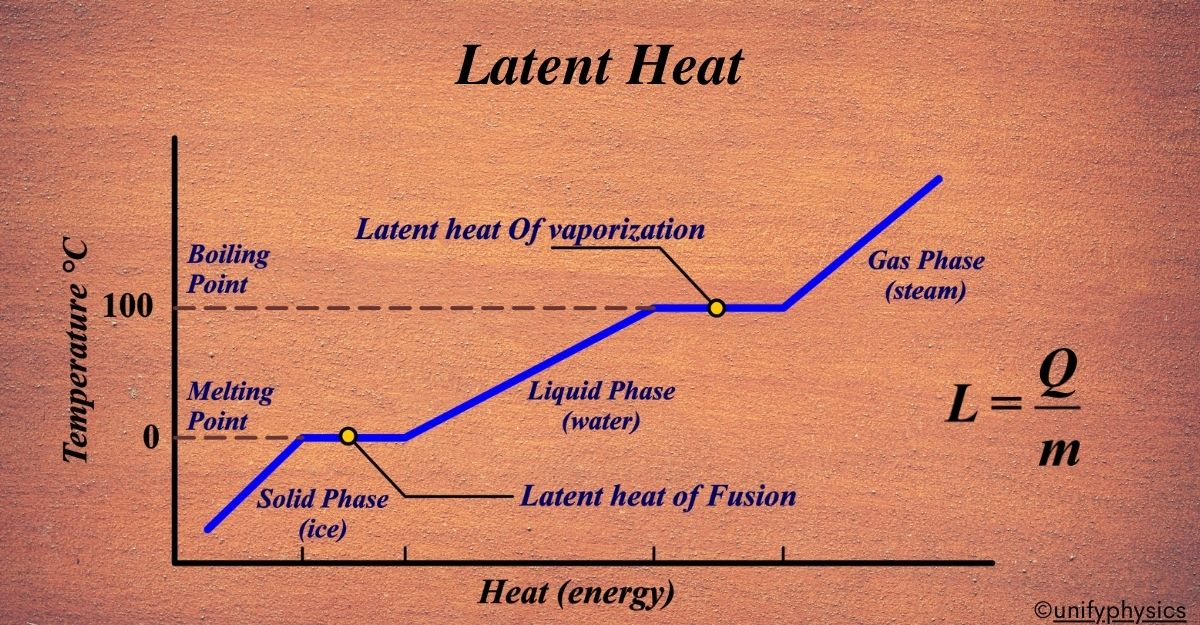
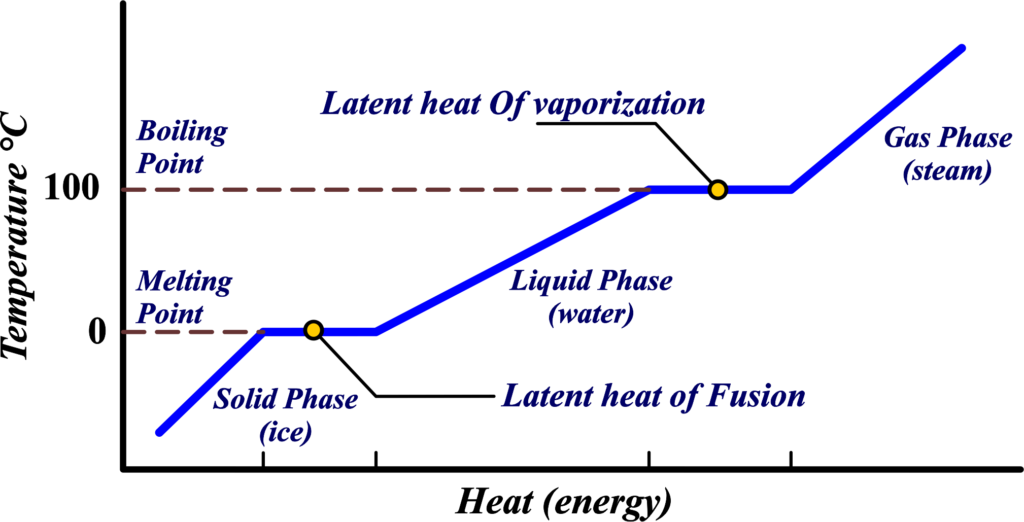
When a solid turns into a liquid, it’s melting, and the energy required for this is called the Latent Heat of Fusion. When a liquid becomes a gas, it’s boiling, and the energy needed here is the Latent Heat of Vaporization. When a solid goes straight to being a gas, bypassing the liquid stage (like dry ice), it’s sublimation, and the energy involved is the Latent Heat of Sublimation.
So, Latent Heat is the undercover energy that’s essential for phase changes, allowing substances to transition between solid, liquid, and gas without altering the temperature. It’s a crucial concept for understanding how energy is stored and released during these transformations.
The formula for Latent Heat
The formula for Latent Heat is quite straightforward. It tells us how much heat energy (Q) is needed to change the phase of a certain mass (m) of a substance without changing its temperature. Here’s the formula:
\(\displaystyle\begin{equation}\label{eqn:1}\boxed{\boldsymbol{ L = \frac{Q}{m}}} \end{equation}\)
- (L) is the Latent Heat,
- (Q) is the heat energy added or removed,
- (m) is the mass of the substance undergoing the phase change.
Unit of Latent Heat
The unit of Latent Heat is derived from the formula itself. Since heat energy (Q) is measured in Joules (J) and mass (m) in kilograms (kg), the unit for Latent Heat (L) is Joules per kilogram (J/kg).
Also Read: Heat
Types of Latent Heat
Latent heat is the heat energy absorbed or released by a substance during a change of state, such as melting, freezing, vaporization, or condensation, without a corresponding temperature change. There are three main types of latent heat: latent heat of fusion, latent heat of sublimation, and latent heat of vaporization.
Latent Heat of Fusion
Imagine you have a solid block of ice. It’s cold and hard because the water molecules are locked in place, holding each other tightly. Now, you want to turn this ice into water without changing its temperature. This is where the Latent Heat of Fusion comes into play.
The Latent Heat of Fusion is like a key that unlocks the molecules from their solid state. It’s the amount of energy you need to provide to the ice so that the water molecules can wiggle free and start flowing as liquid water. This energy doesn’t make the ice hotter; instead, it breaks the molecular bonds holding the water molecules in a rigid structure.
For water, the Latent Heat of Fusion is quite high, which means it takes a lot of energy to melt ice. This is why ice doesn’t instantly melt when you take it out of the freezer. You need to give it time to absorb enough energy from the surroundings to overcome the Latent Heat of Fusion.
In technical terms, the Latent Heat of Fusion (Lf) is the heat required to change 1 kilogram of a substance from solid to liquid at atmospheric pressure without changing its temperature. For water, this value is about ( 334,000 ) Joules per kilogram (J/kg), which is quite a bit of energy!
So, when you’re holding an ice cube, and it starts to melt, that’s the Latent Heat of Fusion at work. The ice cube absorbs energy from your hand (which is warmer than the ice), and this energy goes into breaking the bonds between the water molecules, turning the ice into water. All this happens while the temperature of the ice-water mixture remains at 0°C, the melting point of ice.
Latent Heat of Vaporization
Imagine you’re boiling water to make tea. You turn on the stove, and the water starts getting hotter. But once it reaches its boiling point, something interesting happens: the water temperature stops rising, even though you’re still adding heat. This is where the Latent Heat of Vaporization comes into play.
The Latent Heat of Vaporization is the energy required to transform a liquid into a gas at its boiling point. This energy doesn’t increase the temperature; instead, it breaks the bonds between the liquid molecules, allowing them to spread out and become a gas.
For water, this happens at 100°C (212°F) at sea level. The Latent Heat of Vaporization for water is quite high, which means it takes a lot of energy to turn water into steam. This is why boiling water takes a while before it starts to evaporate.
In technical terms, the Latent Heat of Vaporization (Lv) is the heat required to change 1 kilogram of a liquid into a gas without a temperature change. For water, this value is approximately ( 2.26 × 106 ) Joules per kilogram (J/kg).
So, when you’re waiting for your tea to be ready, the energy you’re adding to the water as it boils is going into overcoming the Latent Heat of Vaporization. It’s the energy needed to give the water molecules enough freedom to move from a liquid state to a gaseous state. And all this happens while the water remains at the boiling point temperature.
Latent Heat of Sublimation
The Latent Heat of Sublimation is a bit like a magic trick in the world of physics. It’s the energy required for a substance to change directly from a solid to a gas without ever becoming a liquid in between. This might seem like a leap, but some substances can do this under the right conditions.
Think of dry ice, which is solid carbon dioxide. If you’ve ever seen dry ice, you know it doesn’t melt into a puddle like regular ice; instead, it turns directly into a foggy gas. This process is sublimation, and the energy that powers this transformation is the Latent Heat of Sublimation.
In technical terms, the Latent Heat of Sublimation (Ls) is the amount of heat needed to convert a unit mass of a solid directly into a gas at a constant temperature. This value is unique for each substance and represents the energy needed to overcome the forces holding the solid together, as well as the energy required to spread the molecules out into a gaseous state.
For example, the Latent Heat of Sublimation for dry ice is quite high, which means it takes a significant amount of energy for the solid carbon dioxide to turn into carbon dioxide gas. This energy goes into breaking the bonds between the molecules in the solid and giving them enough freedom to move around as a gas.
So, when you see a substance like dry ice sublimating, remember that the Latent Heat of Sublimation is the hidden energy at work, allowing the solid to skip the liquid phase and go straight to being a gas. It’s a fascinating phenomenon that shows just how dynamic the interactions between energy and matter can be!
Specific Latent Heat
The term “Specific Latent Heat” refers to the amount of heat energy required to change the state of a specific mass of a substance without changing its temperature. It’s like a personalized energy quota for each kilogram of material.
To understand this, let’s use an analogy. Imagine you have a group of people (representing molecules) in a room (representing the substance). These people are sitting down (solid state), and you want them to stand up (liquid state) without making the room hotter or colder. The energy needed to get one person to stand up is like the Specific Latent Heat for that person. Now, if you want to know how much energy it takes to get a whole kilogram of people to stand up, that’s the Specific Latent Heat for the substance. In more scientific terms, Specific Latent Heat (l) is calculated using the formula:
\(\displaystyle l = \frac{Q}{m} \)
So, if you have 1 kg of ice, and you want to melt it, you’ll need about (334,000) Joules of energy because the Specific Latent Heat of fusion for ice is (334,000) J/kg. This energy will go into breaking the bonds between the ice molecules to turn them into liquid water, all while keeping the temperature at 0°C, the melting point of ice.
Sensible Heat
Sensible Heat is the type of heat that we can feel and measure with a thermometer. It’s the heat that causes a change in the temperature of a substance without changing its phase (from solid to liquid or liquid to gas).
To understand Sensible Heat, imagine you’re heating a pot of water on the stove. As the water heats up, you can see the temperature rise on a thermometer. This increase in temperature is due to Sensible Heat. The heat energy is transferred to the water molecules, making them move faster and faster, which raises the temperature. The formula for calculating Sensible Heat (Q) is:
\(\displaystyle Q = m \cdot c \cdot \Delta T \)
The unit of Sensible Heat in the International System of Units (SI) is the joule (J). In the context of air conditioning, for example, Sensible Heat refers to the heat required to raise or lower the temperature of the air without changing its humidity level.
So, Sensible Heat is all about temperature changes that you can sense. Unlike Latent Heat, which is involved in phase changes, Sensible Heat is the heat you feel when you touch something warm or when you feel the air getting cooler as the AC runs. It’s an everyday experience, like feeling the warmth of the sun on your skin or noticing that a cup of coffee has cooled down to room temperature.
Solved Examples
Problem 1: Calculate the heat required to melt 200 g of ice at 0°C into water at 0°C. Given the latent heat of the fusion of ice is 334 J/g.
Solution:
To melt ice, we use the latent heat of fusion:
\(\displaystyle Q = m \cdot L_f \)
- (m) = 200 g
- (Lf) = 334 J/g
Calculation:
\(\displaystyle Q = 200 \cdot 334 \)
Q = 66800 J
The heat required to melt 200 g of ice at 0°C into water at 0°C is 66800 J.
Problem 2: Find the heat required to convert 150 g of water at 100°C to steam at 100°C. Given the latent heat of vaporization of water is 2260 J/g.
Solution: To vaporize water, we use the latent heat of vaporization:
\(\displaystyle Q = m \cdot L_v \)
Where:
- (m) = 150 g
- (Lv) = 2260 J/g
Calculation:
\(\displaystyle Q = 150 \cdot 2260 \)
Q = 339000 J
The heat required to convert 150 g of water at 100°C to steam at 100°C is 339000 J.
Problem 3: Calculate the total heat required to sublime 50 g of dry ice (solid CO₂) at -78.5°C directly to gas at -78.5°C. The latent heat of sublimation of CO₂ is 571 J/g.
Solution: To sublime CO₂, we use the latent heat of sublimation:
\(\displaystyle Q = m \cdot L_s \)
Where:
- (m) = 50 g
- (Ls) = 571 J/g
Calculation:
\(\displaystyle Q = 50 \cdot 571 \)
Q = 28550 J
The heat required to sublime 50 g of dry ice at -78.5°C is 28550 J.
Problem 4: A student melts 100 g of metal at its melting point, which absorbs 1200 J of heat. Calculate the specific latent heat of fusion of the metal.
Solution: Specific latent heat is given by:
\(\displaystyle L_f = \frac{Q}{m} \)
Where:
- (Q) = 1200 J
- (m) = 100 g
Calculation:
\(\displaystyle L_f = \frac{1200}{100} \)
\(\displaystyle L_f = 12 \, J/g \)
The specific latent heat of fusion of the metal is 12 J/g.
Problem 5: Calculate the heat required to raise the temperature of 250 g of water from 20°C to 80°C. The specific heat capacity of water is 4.18 J/g°C.
Solution: To calculate sensible heat, use the formula:
\(\displaystyle Q = m \cdot c \cdot \Delta T \)
Where:
- (m) = 250 g
- (c) = 4.18 J/g°C
- (\(\displaystyle\Delta T\) ) = 80°C – 20°C = 60°C
Calculation:
\(\displaystyle Q = 250 \cdot 4.18 \cdot 60 \)
Q = 62700 J
The heat required to raise the temperature of 250 g of water from 20°C to 80°C is 62700 J.
Problem 6: Calculate the total heat required to heat 100 g of ice from -10°C to steam at 100°C. Given: specific heat of ice = 2.1 J/g°C, latent heat of fusion = 334 J/g, specific heat of water = 4.18 J/g°C, latent heat of vaporization = 2260 J/g.
Solution:
Heat to raise the temperature of ice from -10°C to 0°C:
\(\displaystyle Q_1 = m \cdot c_{ice} \cdot \Delta T \)
\(\displaystyle Q_1 = 100 \cdot 2.1 \cdot (0 – (-10)) \)
\(\displaystyle Q_1 = 100 \cdot 2.1 \cdot 10 \)
\(\displaystyle Q_1 = 2100 \, J \)
Heat to melt the ice at 0°C to water at 0°C:
\(\displaystyle Q_2 = m \cdot L_f \)
\(\displaystyle Q_2 = 100 \cdot 334 \)
\(\displaystyle Q_2 = 33400 \, J \)
Heat to raise the temperature of water from 0°C to 100°C:
\(\displaystyle Q_3 = m \cdot c_{water} \cdot \Delta T \)
\(\displaystyle Q_3 = 100 \cdot 4.18 \cdot (100 – 0) \)
\(\displaystyle Q_3 = 100 \cdot 4.18 \cdot 100 \)
\(\displaystyle Q_3 = 41800 \, J \)
Heat to convert water at 100°C to steam at 100°C:
\(\displaystyle Q_4 = m \cdot L_v \)
\(\displaystyle Q_4 = 100 \cdot 2260 \)
\(\displaystyle Q_4 = 226000 \, J \)
Total heat required:
\(\displaystyle Q_{total} = Q_1 + Q_2 + Q_3 + Q_4 \)
\(\displaystyle Q_{total} = 2100 + 33400 + 41800 + 226000 \)
\(\displaystyle Q_{total} = 303300 \, J\)
The total heat required to heat 100 g of ice from -10°C to steam at 100°C is 303300 J.
FAQs
What is latent heat?
Latent heat is the amount of energy in the form of heat that is needed to change the state of a substance without changing its temperature. It’s like the hidden energy that helps water turn into ice or steam without getting hotter or colder.
Why is it called ‘latent’ heat?
The term ‘latent’ means hidden. In this context, it refers to the heat energy that is not observed as a temperature change. Instead, this energy is used to change the state of a substance, such as melting or boiling.
Are there different types of latent heat?
Yes, there are two main types: the latent heat of fusion and the latent heat of vaporization. The first one is involved in melting and freezing, while the second one is involved in boiling and condensing.
How does latent heat affect our daily life?
Latent heat plays a role in many everyday processes, like the melting of ice in your drink, the boiling of water for tea, and even the functioning of refrigerators and air conditioners.
Can we measure latent heat?
Scientists measure latent heat to understand how much energy is required for a substance to change its state. This measurement is crucial in many scientific and industrial processes.
Does latent heat involve a change in the internal energy of a substance?
Yes, when latent heat is absorbed or released, the internal energy of a substance changes. This energy goes into rearranging the molecules during the change of state.
What role does latent heat play in weather and climate?
Latent heat is significant in weather and climate as it’s released or absorbed during the formation of clouds and rain. It’s also a key factor in the transfer of energy in the Earth’s atmosphere.