The First Law of Thermodynamics didn’t just appear out of thin air; it was the result of many scientists’ work over several years. It’s like a puzzle that was slowly pieced together by different minds across the world.
Before the First Law was formulated, people believed in the caloric theory, which suggested that heat was a fluid that flowed from hot to cold objects. This idea was popular in the late 18th and early 19th centuries.
Then came a big shift with the work of Benjamin Thompson and James Joule. Thompson conducted experiments that hinted at a relationship between mechanical work and heat. Joule took this further and showed that work could be converted into heat, leading to the idea of the mechanical equivalent of heat.
The formal statement of the First Law, as we know it today, was made by Rudolf Clausius in 1850. He referred to cyclic thermodynamic processes and introduced the concept of internal energy. Around the same time, William Thomson (later known as Lord Kelvin) also contributed significantly to the formulation of the law.
The First Law is essentially a statement about the conservation of energy. It tells us that energy can’t be created or destroyed, only changed from one form to another. This was a revolutionary idea because it meant that all the different forms of energy—mechanical, thermal, electrical, and so on—are interconnected and can be transformed into one another.
What Is the First Law of Thermodynamics?
The First Law of Thermodynamics is a fundamental principle that serves as the bedrock for understanding how energy is used and conserved in physical systems.
The Concept of Energy Conservation
Imagine you have a piggy bank. You can put money in (add energy), or take money out (use energy), but at the end of the day, the amount of money you have is the sum of what you’ve put in and taken out. The First Law says the same thing about energy: the total energy you end up with in a system is the sum of the energy you’ve added and subtracted.
Internal Energy
In thermodynamics, we often talk about the “internal energy” of a system. This is the total energy contained within the system, which includes kinetic energy from the movement of particles and potential energy from the forces between them. When we heat a substance, we increase its internal energy by making its particles move faster.
Heat and Work
The First Law also introduces two ways energy can be transferred: heat and work. Heat is the energy transferred because of a temperature difference, and work is the energy transferred when a force moves an object. In thermodynamics, we’re especially interested in how heat can work, like in an engine or a refrigerator.
First Law of Thermodynamics Equation
The mathematical expression of the First Law is:
\(\displaystyle\Delta U = Q – W\)
Here, (∆U) is the change in internal energy of the system, (Q) is the heat added to the system, and (W) is the work done by the system. If we add heat to a system, its internal energy increases, and if the system does work, its internal energy decreases. If we rearrange this equation, we can also express it as:
\(\displaystyle\begin{equation}\label{eqn:1}\boxed{\boldsymbol{ Q = \Delta U + W}} \end{equation}\)
To understand this equation, think of blowing up a balloon. The air inside (the system) has a certain amount of internal energy. As you blow air into the balloon (adding heat (Q)), the internal energy (∆U) increases. If the balloon expands (doing work (W)), the internal energy decreases because some of the energy you added is now being used to push the balloon’s walls outward.
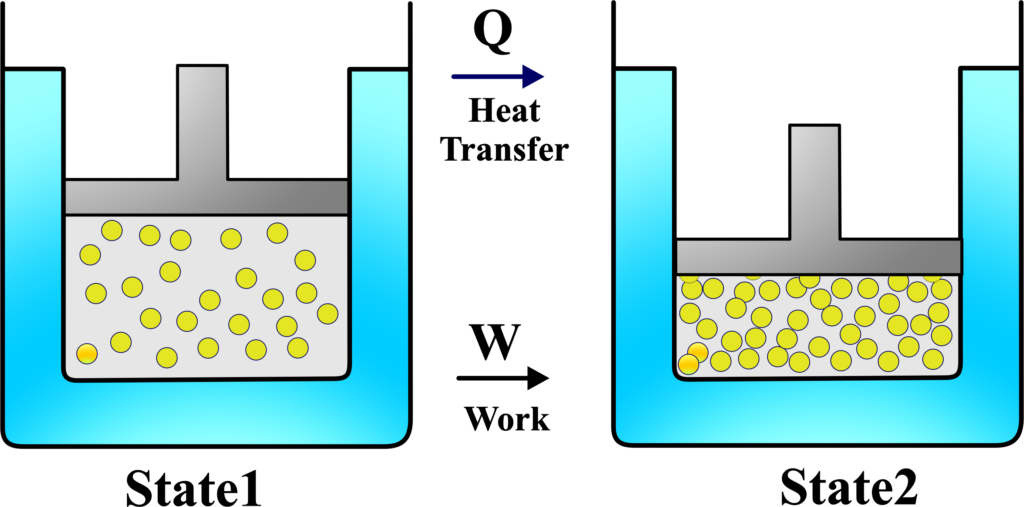
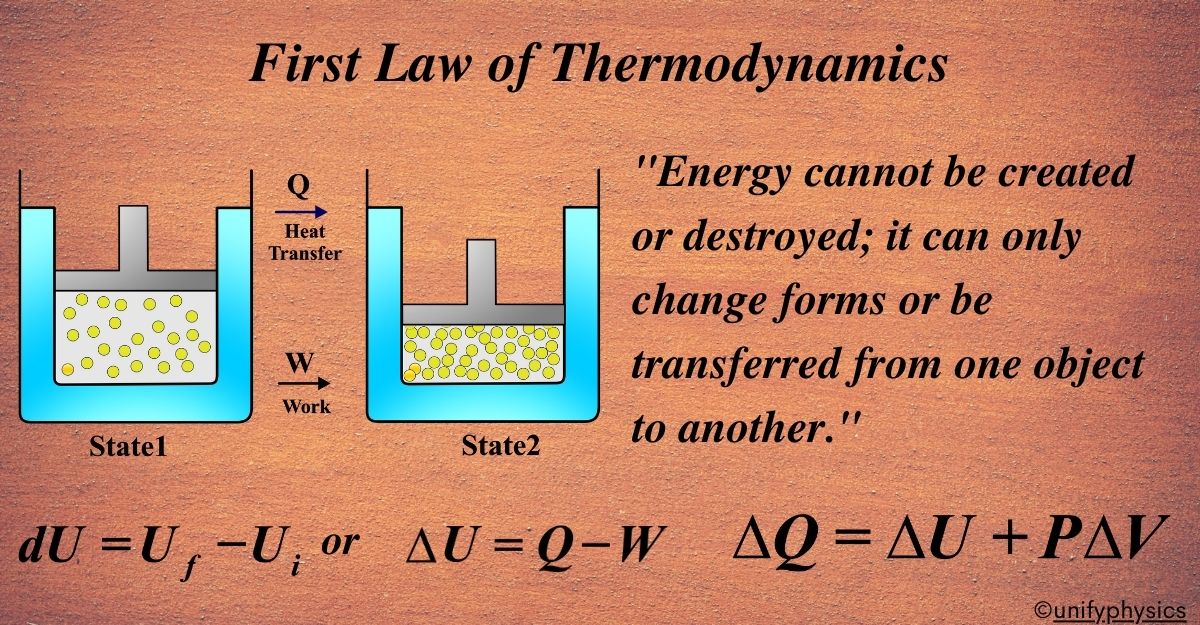
State 1 and State 2 represent two different moments in time for a thermodynamic system. State 1 is before energy is added, and State 2 is after. These particles symbolize the molecules of the system. In State 1, they are less or less energized.
In State 2, there are more particles or they are more energized, indicating an increase in internal energy. The arrow pointing into the system shows heat being added. This increases the system’s internal energy or can cause the system to do work. The arrow pointing outwards from the system indicates work being done by the system, such as moving a piston or turning a wheel.
The First Law of Thermodynamics tells us that the energy added to the system (heat (Q) will increase the system’s internal energy (∆U) and or work done by the system (W ). The equations ( U = Internal Energy) and \(\displaystyle U_{2} – U_{1} = Q – W \) express this relationship, showing that the change in internal energy is equal to the heat added minus the work done by the system.
Derivation
The first law states that the change in internal energy of a system (∆U) is equal to the heat added to the system (Q) minus the work done by the system (W):
\(\displaystyle \Delta U = Q – W \)
Work done by a system in thermodynamics is often the result of a volume change under constant pressure. If we consider work done by the system as the product of pressure and change in volume, we have:
\(\displaystyle W = P\Delta V \)
Replace ( W ) in the first law equation with ( P\Delta V ):
\(\displaystyle \Delta U = Q – P\Delta V \)
To get the desired form, we rearrange the equation to solve for (Q):
\(\displaystyle\begin{equation}\label{eqn:2}\boxed{\boldsymbol{ Q = \Delta U + P\Delta V}} \end{equation}\)
This equation tells us that the heat added to a system is used to increase the system’s internal energy and to do work on the surroundings by expanding against external pressure. It’s a fundamental concept in thermodynamics that helps us understand how energy is conserved and transformed in physical processes. Remember, (∆Q ), (∆U), and (P∆V ) are all state functions, which means they depend only on the initial and final states of the system, not on the path taken to get there.
Limitations
The First Law of Thermodynamics is like a financial ledger that keeps track of energy transactions within a system. It ensures that the energy budget balances out, but it doesn’t tell us everything about the energy’s behavior. While the First Law is powerful, it has its limitations. Here are some of its limitations:
- Doesn’t Predict Direction: The First Law tells us that energy is conserved, but it doesn’t tell us which way the energy will flow. For example, it doesn’t explain why heat flows from hot to cold and not the other way around.
- No Spontaneity Insight: It doesn’t provide any information about whether a process will occur spontaneously. Just because energy conservation is satisfied doesn’t mean the process will happen.
- Efficiency Not Guaranteed: The law states that heat can be converted into work, but it doesn’t guarantee that this will happen efficiently. In reality, some energy is always lost as waste heat, and not all of it can be transformed into useful work.
- Silent on Reversibility: It doesn’t address whether a process is reversible or not. Some processes can go both ways without losing energy, while others can’t. The First Law doesn’t differentiate between these types.
- Doesn’t Account for Energy Quality: All forms of energy are not equal in terms of their ability to do work. The First Law treats all energy the same, but in practice, some forms of energy are more useful than others.
First Law of Thermodynamics for a Closed System
In a closed system, where no matter can enter or leave, the First Law helps us understand how energy changes within the system. For example, if we heat a sealed container, the energy we add doesn’t leave the container; it either increases the internal energy of the gas inside (raising its temperature) or does work by increasing the pressure and potentially doing mechanical work, like moving a piston.
Let’s say you have a sealed container that represents your closed system. If you heat the container, you’re adding energy to it. This added energy can do two things: increase the internal energy of the gas inside (making the molecules move faster and the temperature rise) or do work by pushing on the container’s walls. If the container expands, that’s work being done by the system, and some of the energy you added is used up in doing that.
Sign Conventions
In thermodynamics, we follow certain sign conventions to keep track of energy movement:
- If heat is added to the system, (Q) is positive.
- If work is done by the system, (W) is positive.
- If heat is removed from the system, (Q) is negative.
- If work is done on the system, (W) is negative.
This law is crucial for understanding how engines work, how refrigerators cool food, and even how our bodies use food for energy. It’s a fundamental principle that ensures that in any process within a closed system, energy is neither lost nor appears from nowhere—it’s always conserved.
Formulae
According to the first law of thermodynamics: \(\displaystyle dQ=dU+dW=dU+PdV\)
For a change of state: \(\displaystyle dQ=mL\)
For temperature rise: \(\displaystyle dQ=mC\Delta T\)
Change in internal energy : \(\displaystyle dU={{U}_{f}}-{{U}_{i}}\)
Also Read: Zeroth Law of Thermodynamics
FAQs
Q: What is the First Law of Thermodynamics, and how does it relate to energy conservation?
- Answer: The First Law of Thermodynamics states that energy cannot be created or destroyed, only converted from one form to another. This law emphasizes the principle of energy conservation, stating that the total energy of an isolated system remains constant over time, even as it undergoes various processes and transformations.
Q: Can you explain how the First Law of Thermodynamics applies to heat engines?
- Answer: In heat engines, the First Law of Thermodynamics governs the conversion of heat energy into mechanical work. According to this law, the total energy input to the engine in the form of heat must equal the total energy output in the form of work done and any heat rejected to the surroundings. This principle is essential for analyzing the efficiency and performance of heat engines.
Q: How does the First Law of Thermodynamics relate to internal energy changes in a system?
- Answer: The First Law of Thermodynamics relates to changes in the internal energy of a system by stating that any change in internal energy is equal to the heat added to the system minus the work done by the system on its surroundings. This relationship helps quantify the energy changes associated with various thermodynamic processes.
Q: What role does the First Law of Thermodynamics play in understanding chemical reactions?
- Answer: In chemical reactions, the First Law of Thermodynamics governs the conservation of energy. It states that the total energy of the reactants must equal the total energy of the products, accounting for any heat absorbed or released during the reaction. This principle is fundamental for analyzing reaction kinetics, enthalpy changes, and reaction energetics.
Q: How does the First Law of Thermodynamics apply to adiabatic processes?
- Answer: In adiabatic processes, there is no heat transfer between the system and its surroundings, so the First Law of Thermodynamics simplifies to (∆U = -W ), where (∆U) is the change in internal energy and (W) is the work done by the system. This relationship helps characterize the energy changes in adiabatic processes, such as in adiabatic compression or expansion of gases.
Q: Can the First Law of Thermodynamics be violated?
- Answer: No, the First Law of Thermodynamics is a fundamental principle of physics and cannot be violated. It states that energy is conserved in all processes, meaning the total energy of an isolated system remains constant. Any apparent violations would indicate an error in measurement or an incomplete understanding of the system under consideration.
Q: How does the concept of heat transfer contribute to the understanding and application of the First Law of Thermodynamics?
- Answer: Heat transfer is central to the application of the First Law of Thermodynamics, as it represents one of the primary mechanisms by which energy is exchanged between a system and its surroundings. By quantifying heat transfer and accounting for any work done, the First Law helps explain and predict the behavior of thermodynamic systems undergoing various processes.